Catalytic Tools for Carbon-Carbon Bond Applications
A classic reaction for creating carbon-carbon bonds was revisited in order to minimize its demand for magnesium through modern, catalytic pathways.
.
Carbon-carbon bonds are fundamental in organic chemistry. Forming and cleaving of carbon-carbon bonds is involved in numerous reactions. The Grignard addition reaction, discovered by Victor Grignard in 1900, is a commonly employed method for creating carbon-carbon bonds. In this reaction however, a stoichiometric amount of magnesium is required. This is not unfeasible in itself, since magnesium is an abundant element, and not too costly. Still, it would be desirable if modern, catalytic pathways could minimize this relatively large demand for magnesium. In the project, the Grignard reaction was revisited for this purpose. The results are hoped to also be relevant for other types of catalyzed reactions. In some of these, stoichiometric amounts of catalyst would be unacceptable due to the high cost of the catalyst material.
The Grignard reaction is an example of organometallic chemistry, which is an overlapping field of inorganic and organic chemistry, since it involves bonds between a metal and carbon. The reaction uses organometallic substances known as Grignard reagents. A Grignard reagent is a magnesium halide bound to a carbon compound. It has to be prepared prior to the addition step. The addition must be performed under strictly anhydrous conditions in ethereal solvents (usually diethyl ether or tetrahydrofuran). Grignard reagents react with a great variety of carbonyl compounds, including aldehydes, ketones, formaldehyde, esters, and amides.
Revisiting the Grignard reaction, this benzyl addition reaction was found to be a reversible transformation. The retro benzyl addition was shown by the addition of benzylmagnesium chloride to di-t-butyl ketone followed by exchange of both the benzyl and the ketone moiety with another substrate.
Ring-opening of cyclic ethers with concomitant C-C bond formation was studied with a number of Grignard agents. The transformation was performed in a sealed vial by heating to about 160 °C in an aluminum block or at 180 °C in a microwave oven. Good yields of the product alcohols were obtained with allyl- and benzylmagnesium halides when the ether was tetrahydrofuran or 3,3-dimethyloxetane.
Carbohydrates with protecting groups on all alcohol groups except the primary alcohol were prepared and subjected to the iridium catalyzed dehydrogenative decarbonylation reaction where primary alcohols are converted into the corresponding one carbon shorter products.
The syngas evolved from the iridium catalyzed dehydrogenative decarbonylation reaction was consumed in a palladium catalyzed reductive carbonylation reaction in a two-chamber system setup. Of the simple primary alcohols investigated, 2-(2-naphthyl)ethanol, hexane- 1,6-diol and dodecane-1,12-diol were found to be the most promising syngas sources. A substrate scope for the reductive carbonylation of aryl bromides is currently under development with hexane-1,6-diol as syngas source.
The synthesis of the anticancer antibiotic tetrahydroisoquinoline alkaloid jorumycin progressed via a route consisting of a crucial aryne annulation step where an isoquinoline scaffold was prepared. A more promising, alternative route was also identified.
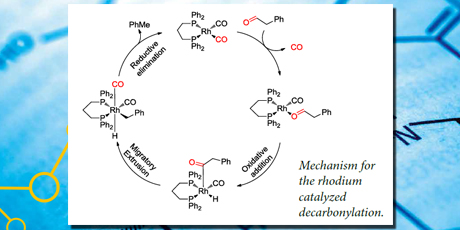
Caption: Mechanism for the rhodium catalyzed decarbonylation.