Characterization of Blood Coagulation Protein Complexes
Characterization of protein complexes involved in the blood coagulation cascade can lead to improved treatment for haemophilic patients.
Up until a certain point, the blood is capable of repairing traumatic injury and stop bleedings. The network of inter-connected biochemical coagulation reactions which occurs upon vessel injury is called haemostasis. Insufficient ability of the blood to coagulate is called haemophilia. The thesis focuses on characterization of protein complexes involved in the blood coagulation cascade.
More specifically, molecular dynamics simulations have been performed to elaborate in atomistic detail intricate structural and functional relationships of constituting components of the two principal factor X-activating protein complexes in the cascade. Such insights can lead to development of improved treatment for haemophilic patients. The results are also of relevance for anti-coagulant treatments for patients with the reverse problem, namely too strong coagulation – thrombosis.
Roughly 400,000 males around the world suffer from haemophilia. Symptoms include prolonged bleedings, joint bleedings, muscle and sub-cutaneous bleedings, internal bleedings, and inter-cranial bleedings. The disease can be treated, with NovoSeven® being one of the leading products for treatment of patients with inhibitory antibodies against FVIII or FIX.
The number “seven” in the name of the product refers to the coagulation factor VII (FVII), or more precisely FVIIa. Dosing of a recombinant form of this coagulation factor can provide haemostasis in patients with severe haemophilia A or B, offering a safe treatment without significant side-effects.
In the project, the complex between FVIIa and Tissue Factor (TF) was studied. It was shown that TF could preserve the productive conformation of the activation domain in FVIIa. This decisive result suggests for the first time an allosteric mechanism involving the E2 strand-following segment acting as a gatingresidue for the active site cleft. While it is long established that FVIIa binds TF in an extended conformation, it has not previously been shown how this extension is related to the flexibility of the said linker.
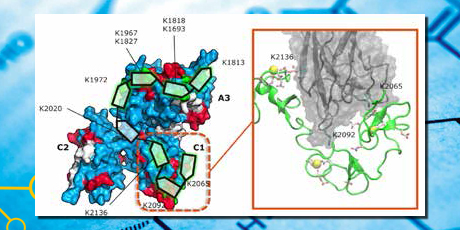
The membrane-bound states of the co-factors FVIII and FIX are important for their function. The resulting conformations of the membrane-anchoring domains from FVIII were studied. Surprisingly, FVIII C1 and FVIII C2 did not converge to similar distribution of membrane-bound states. Lastly, an illustrative canonical binding mode between the LRP receptor and FVIII as ligand was constructed.
Overall, the studies presented have added structural insight at the atomistic scale and a dynamical perspective on FVII activation and FVIII trafficking.
Caption: Canonical (triplet) binding mode.