Ionic Liquid Catalysts for Biomass Conversion
Two ionic liquid catalyst systems were used to catalyze dehydration of glucose into HMF, which is an important building block in biorefineries.
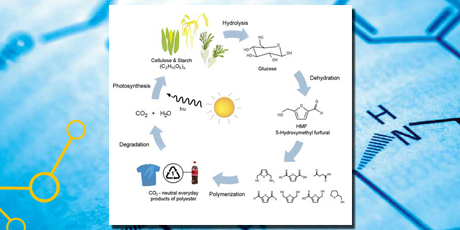
Generation of chemicals from biomass is subject to increased interest. The use of biomass feedstock instead of fossil raw materials is desirable both due to the fact that biomass is a renewable source, and climate considerations. Biomass is CO2 neutral in the sense that the CO2 released would have been taken up by plants during their growth. One approach is to build a platform of chemicals that are less used today, but potentially easily obtained from biomass, and which could replace other chemicals that are produced from fossil raw materials. The thesis focusses on catalytic processes that enable production of such platform chemicals.
HMF (5-Hydroxymethylfurfural) can be made from sugars and further processed into a range of chemicals, which can be used for production of polymers or into DFF (2,5-diformylfuran), which is the starting material for various medical and cosmetic products. In the project glucose (which can be obtained from biomass) was converted into HMF.
More specifically, two ionic liquid catalyst systems with either Cr(II) chloride or Cr(III) chloride, were used to catalyze dehydration of glucose into HMF. The system was investigated via several spectroscopic techniques to observe the catalytically active sites. It was shown that during the catalytic reaction, a species of the form [CrCl4(OR)2]- was formed from [CrCl6]3- in the solution. These are the predominant chromium containing species in the solution.
Another group of interesting platform chemicals is aldehydes. For the first time in the reported literature, ionic liquids were shown to be viable solvents for the homogeneous decarbonylation of aldehydes. [Rh(dppp)2]Cl (dppp = 1,3-bis(diphenylphosphino)propane) was used as catalyst. Decarbonylation of both aromatic and aliphatic aldehydes proved possible to an excellent extent. A series of ionic liquids were tested. The best system was comprised of [Rh(dppp)2]Cl in [BMIm]Cl (1,3-butylmethylimidazolium chloride). It was shown that the ionic liquid stabilized the catalytic system to a degree where the reuse of the catalyst was possible for three cycles while maintaining good catalytic activity (99-85 %). The catalytic reaction yielded a biphasic system, and therefore the separation of the product from the reaction mixture was done with ease, showing the benefits of this system compared to traditional organic solvents.
Finally, [Rh(dppp)2]Cl impregnated on a silica support was used for heterogeneous decarbonylation of aldehydes in a continuous flow setup. The catalyst showed yields up to 95 % at elevated temperature and both aromatic and aliphatic aldehydes could be decarbonylated. This setup could be a novel way to run industrial decarbonylation.
Capture: The use of biomass in the industry, a representative illustration showing the promise of the valorization of biomass.