Synthesis of Breast-milk Compounds
The main focus is human milk oligosaccharide (HMO) synthesis based on the motif Galβ1-3/4GlcNAcβ1-3Galβ1-4Glc, which is the core of many HMO’s.
For more than a century it has been known that babies fed on breast-milk have lower risk of several diseases compared with bottle-fed infants. While the exact mechanisms are still under debate, this difference has spurred interest in adding key components from human milk to infant formula, which is commonly produced from bovine milk. Human milk contains a range of biomolecules including vitamins, enzymes, proteins, and oligosaccharides. The main focus of the thesis is human milk oligosaccharide (HMO) synthesis while a second and distinct part is dedicated to a protein relevant for future cancer treatment. Both topics are related to carbohydrate and organometallic chemistry.
HMO’s are the third most abundant component in breast-milk (5-15 g/L). HMO’s are composed of five monosaccharide building blocks: galactose (Gal), glucose (Glc), N-acetylglucosamine (GlcNAc), fucose (Fuc), and N-acetylneuraminic acid (Neu5Ac). In bovine milk oligosaccharides are less abundant and structurally less complex than HMO.
The synthesis is based on the motif Galβ1- 3/4GlcNAcβ1-3Galβ1-4Glc, which is the core of many HMO’s. Three distinct HMO’s were synthesized: Lacto-N-tetraose, Lacto-N-fucopentaose I, and Lacto-N-neofucopentaose I.
A one-pot strategy was developed for synthesis of the tetrasaccharide backbone core based on the different reactivity of thioglycoside donors and acceptors. Deprotection of the protecting group at the C-2-position on the galactose moiety liberated an acceptor for the fucosylation eventually creating the two linear pentasaccharides Lacto-N-fucopentaose I, and Lacto-N-neofucopentaose I.
The scope of the developed one-pot method was enhanced by 1-4 glucosylations utilizing a glucosamine building block containing two free hydroxyl groups. In addition, pNP-neuraminic acid was synthesized for enzyme activity studies. The enzymes were designed to perform sialyl transfer reactions in HMO’s containing neuraminic acid.
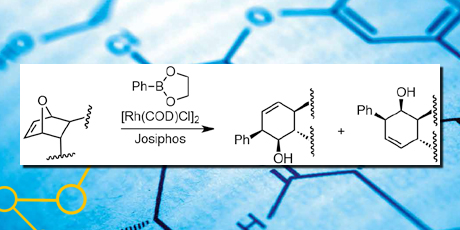
The second and distinct part of the thesis describes regio-selective ring opening of enantiopure oxabicycles primarily by use of rhodium catalysts and phosphine ligands. The ring opened products were similar to compounds shown to be potential protein Bcl-XL antagonists, a target for future drugs in cancer treatment. By employing a [Rh(COD) Cl]2 catalyst with a Josiphos ligand, it was possible to perform the ring opening of oxabicycles with ester moieties in good yield and excellent regio-selectivity.