Catalysis in Sustainable Chemistry
An atom-economical way of synthesizing esters by dehydrogenative
coupling of primary alcohols, catalyzed by a ruthenium complex is presented.
The concept of ”atom economy” in chemistry was introduced by Trost in 1991. The basic idea is to maximize the share of reactant atoms that can be found in the end product(s), thus minimizing waste. In practice this often depends on catalysts which assist by arranging the atoms during the process. Efficient catalysts therefore play a key role in sustainable chemistry. More specifically, a number of transition metals have proved to be highly efficient catalysts. The thesis investigates two classes of transition metal catalyzed reactions with large practical applications, and one sequence of mainly theoretical importance. The last few years have seen a steep rise in the interest in dehydrogenative reactions in the scientific literature. These reactions are atom-economical in the sense that the only redundant atoms are hydrogens which are cleaved off, producing molecular hydrogen as the only byproduct. The thesis presents a new atom-economical way of synthesizing esters by dehydrogenative coupling of primary alcohols, catalyzed by a ruthenium NHC complex. Esters are produced industrially on a very large scale.
The proposed reaction is catalyzed by the ruthenium N-heterocyclic carbene complex RuCl2(IiPr)(p-cymene). The optimal reaction conditions were found to be 2.5 mol % of the carbene complex, 4.5 mol % of PCy3 and 10 mol % of KOH in refluxing mesitylene. The substrate scope was shown to include a range of different straight-chain and branched primary alcohols, which reacted to give the corresponding esters in moderate to excellent yields. Condensation of diols also proceeded well, giving the corresponding lactones in good yield. Preliminary investigations confirm that the reaction is dehydrogenative. A catalytic cycle is proposed.
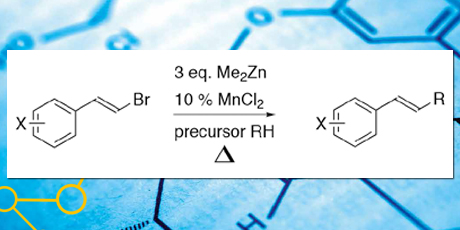
Another area of high attention is the idea of replacing palladium with alternative catalysts. Palladium is a highly efficient catalyst in many contexts, but is unfortunately both expensive and toxic, which limits its use for a number of catalytic applications. Finding cheaper and less toxic alternatives is highly desirable. The thesis presents a new method for formation of styryl derivatives by reaction of ether and hydrocarbon radicals with β–bromostyrenes. The best conditions were found to involve addition of three to four equivalents of Me2Zn to a solution of β–bromostyrene, using the radical precursor as solvent, in the presence of 10-12 % of MnCl2, and refluxing overnight by the presence of air. A simple acidic workup and purification by chromatography gave the products in moderate to good yield.
Finally, a new member of the family of [5]-phenylenes, named Anti Zigzag-[5]- phenylene, was synthesized and characterized. The molecule was synthesized in ten steps, of which six steps had not been performed before, and six new compounds were isolated and characterized. The molecule is primarily of theoretical interest. Its complex synthesis underlines the unrivaled capacities that can be achieved by practical application of transition metal catalyzed reactions.