Anti-cancer Drugs Based on Epigenetic Modulation (II)
The thesis presents synthesis pathways for a number of histone deacetylases (HDAC) inhibitors of which several are synthesized for the first time.
Histone deacetylases (HDACs) are a family of enzymes, which serve as epigenetic modulators. Epigenetics is defined as the processes that regulate gene expression in cells without changing the DNA sequence. The function of HDACs is related to DNA transcription and regulation of various biochemical pathways. It is established that HDACs are relevant targets for anti-cancer drugs, and several HDAC inhibitors are already either approved medicines or undergoing clinical trials. The thesis presents synthesis pathways for a number of HDAC inhibitors of which several are synthesized for the first time.
Macrocyclic peptides and depsipeptides constitute an interesting class of HDAC inhibitors. These compounds are found in nature, and they are highly potent and moderately selective HDAC inhibitors. The focus of the preent thesis was a sub-class of these substances, namely cyclic tetrapeptides known as azumamides.
A synthetic route was developed, which allowed total synthesis of azumamides A–E. This is the first reported total syntheses of azumamides B–D.
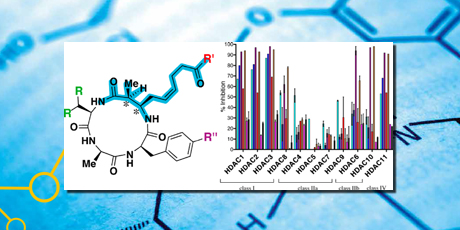
The key step in this route was a diastereoselective Mannich reaction, which enabled preparation of two site-specifically edited epimeric azumamide analogs, where the stereochemistry in the unique β-amino acid was inverted. The two epimeric homologs were tested together with azumamide A–E against the entire panel of recombinant HDAC isoforms, providing the first full profiling of the azumamides. It was shown that the β-amino scaffold is highly sensitive to stereochemical modifications.
The profiling of the natural products showed that the azumamides are poor inhibitors of certain HDACs (class IIa) and potent inhibitors of others (HDAC1-3, 10, and 11 – with IC50 values between 14–67 nM). Furthermore, carboxylic acid containing compounds (azumamide C and E) were more potent than their carboxyamide counterparts (azumamide A and B).
Isoform selectivity was observed in class I and class IIb. In class I, azumamides C and E were 60–350 times more potent towards HDAC1-3 over HDAC8, and in class IIb they were ›200-fold more potent against HDAC10 over HDAC6. Finally, azumamide C was approximately twice as potent as azumamide E, which indicates that having a tyrosine residue in the macrolactam ring increases the activity compared with the phenylalanine homolog. The synthetic route was elaborated to produce structurally edited azumamide analogs. A series of β2-desmethylated compounds were synthesized in parallel to a series of β2-dimethylated analogs, and a tryptophan-containing series was also prepared.
In conclusion, the project has contributed to understanding of the structure-activity relationship for the azumamides. It was illustrated that these compounds are particularly sensitive to modifications in the β-amino acid residue. The developed diastereoselective Mannich reaction has been shown to be a powerful tool for synthesizing β-amino acid scaffolds, and this reaction will aid in future production of azumamide analogs.