Nano-structured Catalysts for Green Chemistry
A novel method for the two-step synthesis of amides from alcohols and amines using Au/TiO2 nanoparticles and base as catalysts is presented.
Catalysis aids the conversion of raw materials into valuable chemicals, materials and fuels in an efficient and environmentally benign manner. Catalytic processes are therefore essential to the industry and to the world economy. Catalysts enhance the rate of chemical reactions, often by many orders of magnitude. Further, catalysts are also important because of their selectivity, i.e. their ability to increase the rate of formation of one particular reaction product relative to other possible, but undesired by-products. In the project, a number of nano-structured catalysts have been synthesized and characterized.
Gold (Au) nanoparticles are particularly interesting. While bulk gold is chemically inert, supported gold nanoparticles can promote several reactions under mild conditions.
A novel method for the two-step synthesis of amides from alcohols and amines using Au/ TiO2 and base as catalysts is presented. In the first step, a methyl ester is obtained by goldcatalyzed aerobic oxidation of the alcohol in methanol. In the second step, amine is added and the methyl ester undergoes base-catalyzed aminolysis to give the desired amide. As the same base promotes both reactions, the synthesis can be performed in a one-pot procedure. The reaction protocol was applied to a number of different alcohols and amines, demonstrating the versatility of the method.
A Hammett study indicated that the goldcatalyzed oxidation of benzyl alcohol occurs with build-up of positive charge in the benzylic position, which corresponds well with a H-abstraction step. The results were in good agreement with the generally accepted perception that the gold catalyzed esterification of alcohols with methanol occurs through an aldehyde intermediate. The rate of amide formation was seen to be very sensitive to substituent effects, and negative charge was formed during the reaction.
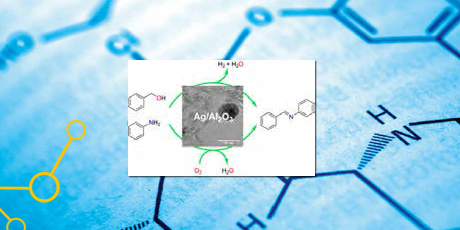
Further, imines were synthesized directly by oxidative coupling of alcohols and amines using different supported silver catalysts. The reactions were performed at relatively mild conditions (100 °C and atmospheric pressure) and afforded the desired imines with high selectivity (up to 99%). The highest catalytic activity was obtained with 5 wt% Ag/Al2O3 in toluene with air as oxidant.
Pyridine was oxidized to pyridine N-oxide using TS-1 and H2O2 as oxidant. Mesoporous TS-1 prepared by carbon templating was significantly more active than conventional TS-1. UV-Vis spectroscopy indicated that desilication causes a surface densification of less active extra-framework Ti species, while carbon-templating is a more gentle and effective method to introduce mesopores.
Finally, a simple and effective method to encapsulate gold nanoparticles into silicalite-1 is presented. The crystals were modified by recrystallization, which creates intra-particle voids and mesopores that facilitated the formation of gold nanoparticles by simple impregnation. Remarkable stability, catalytic activity, and selectivity for gas-phase oxidation of bioethanol to acetaldehyde were demonstrated. This may become a favorable and green alternative to the conventional ethylene route.
While previous attempts to encapsulate metal nanoparticles in zeolites have relied on expensive additives and complex procedures, the presented approach is simple, effective and scalable. The method could thus become a helpful tool in the further development of nanostructured catalysts.
Caption: A green method to synthesis imines by aerobic oxidative coupling of amines and alcohols under mild reaction conditions with Ag/Al2O3 as catalyst was shown.