Towards Nano-scale Electrochemical Sensors
Prussian Blue nanoparticles were synthesized and proven highly efficient in interfacial electrochemical electron transfer and as enzyme mimetic nanoscale electrocatalysts.
Nano-size sensors are attractive for several reasons. They can be highly sensitive, and analyte quantities in principle right down to a single molecule can be detected. Secondly, the space requirements are very small, contributing to lab-on-a-chip technology. The amount of material required for sensor construction is finally small. Focus of the present thesis is how catalytic properties of different types of nanostructures can be utilized in nanoscale chemical and biological sensing. The thesis also contributes to understanding of the fundamental catalytic processes. Surface catalytic reactions are very sensitive to the atomic-level structure of the interface. A core feature of nanostructures in relation to sensing is further their ability to catalyze electrochemical reactions. Different dimensions and types of nanomaterials can enhance electrocatalytic activity, and thus offer to be highly suitable for nanoscale sensing.
Prussian Blue (PB) – Fe4[Fe(CN)6]3 – has been widely used for centuries as a dye. In 1978, Neff showed PB to be electro-active, and in 1984 Itaya discovered that PB can catalyze electrochemical reduction of hydrogen peroxide (H2O2) and O2. Detection of H2O2 is important in many contexts. It is a waste product from nuclear power stations and industry, and monitoring H2O2 in ground water or rain is often relevant. Further, H2O2 is used to disinfect beverage and food packaging, water pools, and in several other applications. Later it has been suggested to use PB mixed with enzymes as a biosensor probe to detect glucose, galactose lactate, cholesterol, choline, and other biological substances.
PB nanoparticles with excellent redox electroactivity have been synthesized in the Ph.D.- project. They proved highly efficient both directly in interfacial electrochemical electron transfer and as enzyme-like nanoscale or even molecular scale electrocatalysts. The particles were immobilized on a single-crystal, atomically planar Au (111) electrode surface modified with functionalized alkanethiol linkers and shown to display highly efficient electrocatalysis towards reduction of H2O2, and a quantitative relationship between the electron transfer kinetics and electrocatalytic activity.
Graphene is a single-atom thick plane of layered graphite. So-called graphene paper was first introduced by Ruoff and co-workers in 2007. Graphene paper doped with PB nanoparticles was prepared and studied in the present Ph.D.- project. The product was shown to have increased electrical conductivity compared with the components, making it a nano-hybrid electrocatalyst of high functional variability. It was shown useful as electrochemical sensor material for detection of H2O2 or organic peroxides. Noting the high stability, low cost and efficient electrocatalysis, the PB-doped graphene paper has potential to be produced in large scale, not only for sensing but also for other applications such as electrocatalytic energy conversion.
A new method for fast fabrication of large-area graphene nanosheets doped with “quantum dots” (2-3 nm CdSe nanoparticles) was finally developed. This type of mixed nanomaterial showed new fine-structure for enhancement of photo-induced electron or energy transfer, and could potentially be used in applications such as photo-voltaic material in solar cells.
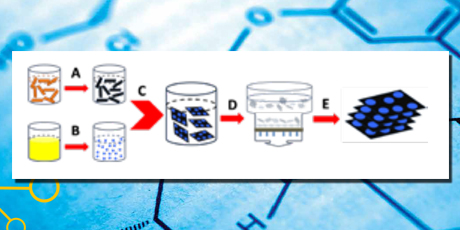
Capture: Schematic illustration of experimental preparation procedures. A) Wet-chemical conversion of graphene oxide (GO) to reduced GO via hydrazine reduction, B) synthesis of PNBPs starting from the mixture of FeCl3 and K4[Fe(CN)6], C) preparation of PBNPs–RGO hybrid nanosheets, and D,E) processes of preparing PBNPs–RGO hybrid paper including filtration, drying and annealing. Not drawn to scale.