Synthesis of Key Plant Cell Wall Components
Methods for synthesizing branched- and linear oligogalactosides have been developed by preparation of three small libraries of oligosaccharides.
The cell walls represent almost half of the biomass found in plants. Understanding the plant cell walls is thus of enormous importance both in regard to economy and sustainability. Examples are the use of plants for transport fuels, functional foods, and raw materials to generate chemical building blocks for industrial purposes. In this thesis, novel methods for the synthesis of a number of key plant cell wall components are presented. The idea is that such structurally well-defined substances can serve as models for the study of the more complex components found in plants. This may aid investigations on i.e. cell wall biosynthesis and protein-carbohydrate interactions.
The cell wall is composed by a network of polysaccharides (approximately 90 %) and protein components (app. 10 %). The primary cell wall is divided into two categories. Type I walls consist of a cellulosic microfibrils cross-linked by hemicelluloses and enmeshed in a non-covalently cross-linked pectin network. Type II walls are organized in essentially the same way but with the matrix mainly composed by glucuronoarabinoxylans (GAXs) and β–D-(1→3; 1→4)-glucans (mixed-linkage glucans, MLGs).
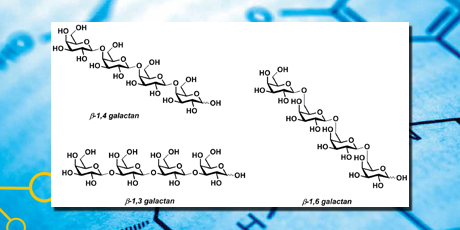
The thesis presents the chemical synthesis of fragments of galactans and arabinogalactans that are prominent side chains of the pectic polysaccharide rhamnogalacturonan I (RG-I) and the main component of arabinogalactan protein (AGP).
A novel, mild procedure for hydrolysis of n-pentenyl glycosides was developed, making it possible to obtain the corresponding PTFAI donors in high yields. These proved to be exceptional donors for glycosylation of pentenyl glycoside acceptors and gave access to fully protected pentenyl disaccharide donors.
A 3,6-O-benzyl protected donor made it possible to prepare a small library of oligogalactans including linear tetra-, penta-, hexa-, and heptasaccharides and six (1→6)-branched hepta- or octasaccharides. The assembly of the oligosaccharides went smoothly with good to excellent yields and high selectivity for the desired β–linked products. This constitutes the first reported synthesis of branched β–(1→4)- linked galactans.
Proof of concept for the utility of the synthetic cell wall fragments was obtained by researchers at Lawrence Berkeley National Laboratory, California, at DTU Systems Biology in Lyngby, and at University of Copenhagen, while further studies are currently undertaken by the German Cancer Research Center in Heidelberg.
In conclusion, methods for synthesizing branched- and linear oligogalactosides have been developed and exemplified by preparation of three small libraries of oligosaccharides. The value of synthetically prepared, well-defined oligosaccharides for plant research has been shown.
Capture: Lipid I biosynthesis and the target Tunicamycin analogue