New Weapons against Multi-resistant Bacteria
Multi-resistant bacteria such as MRSA are an increasing problem. The project has identified several promising antibiotics candidates.
As multi-resistant bacteria are a growing concern worldwide there is a need for new effective antibiotics. Preferably their effect should be due to other mechanisms than traditional antibiotics, which bacteria such as MRSA (Methicillin-resistant Staphylococcus aureus) currently seem to be able to resist. The thesis presents several novel antibiotics candidates.
In recent years interest in bacterial topoisomerase inhibitors (NBTIs) has increased. Their mode of action is distinctively different from the dominant type of antibiotics, the fluoroquinolones. NBTIs target the vital bacterial enzymes DNA gyrase and topisomerase IV. Unfortunately, several NBTIs have been associated with undesired inhibition of the hERG potassium channels in humans, which can lead to cardiac arrhythmia. One NBTI, NXL101, was withdrawn from clinical phase I trials for this reason.
The main focus of the project has been redesign of NXL101 to facilitate synthesis of related NBTIs with the idea of preserving the antibacterial activity without having the undesired hERG inhibition. For this purpose a new piperazine-based NBTI template was developed, and more than 100 analogs were synthesized. The most potent analog displayed higher antibacterial activity against MRSA (and MSSA) than NXL101, while the hERG affinity was decreased. A study showed that no resistance was developed over the course of 22 days, during which time the reference ciprofloxacine developed high resistance (from a MIC = 1.6 to ›25 μg/mL).
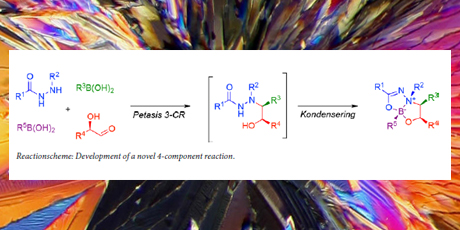
In a second part of the project, strategies to access structurally diverse compound collections were addressed. Firstly, the Petasis 3-component reaction was used for creation of a densely functionalized hydrazido-alcohol template, which was further transformed into an array of skeletally diverse compounds via multiple modes of cyclization. Secondly, a novel 4-component reaction was developed based on the Petasis 3-component reaction employing an additional boronic acid to afford a diversely substituted bicyclic boronate. Thirdly, a strategy for the synthesis of spiro- and macrocycles was devised. The strategy relies on an intramolecular Diels-Alder reaction to create a complex scaffold, incorporating three new stereogenic centers, two of which are quaternary.
Overall, the project gives reason to assume that further molecules displaying desirable pharmacological properties can be found through the outlined strategies, notably via discrete structural modifications of the piperazine-based NBTI template.