Tandem Reactions in Organic Chemistry
By replacing multistep procedures with cooperative catalytic systems, more selective and efficient synthesis can be obtained.
An ideal chemical reaction is efficient, selective and “atom-economical”, meaning that the participating atoms end up in the resulting product(s) to the highest possible degree. By replacing multistep procedures with one-pot catalytic systems, a high degree of selectivity and efficiency can be obtained. Compared to traditional methods, which often generate side products such as salts, catalysts may activate otherwise inaccessible functionalities, thus leading to more efficient and atom-economical reactions. The thesis presents new such catalytic reactions in organic chemistry.
Tandem reactions are consecutive chemical transformations combined into one synthetic operation. The idea is to achieve a cooperative catalytic system. When subjecting an appropriate substrate to the system, the capability of two catalysts can be exploited in an efficient manner, resulting in remarkable transformations. Optimization of reagent compatibility is often required in order to succeed.
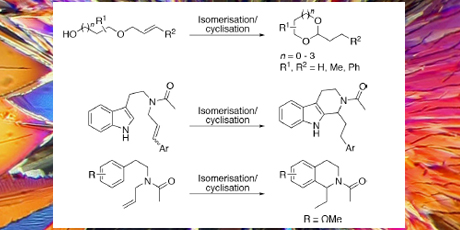
In the project a tandem isomerization/cyclisation reaction sequence initiated by a dual transition metal/Brønsted acid catalytic system has been developed. The methodology was applied on allylic ethers and amides where the transition metal catalyst initiates olefin migration to the corresponding enol ether or enamide. Subsequent Brønsted acid catalyzed tautomerisation results in oxocarbenium and N-acyliminium ions, which are trapped by tethered nucleophiles. Cyclic acetals, tetrahydro- β-carbolines and tetrahydroisoquinolines have been synthesized through this tandem reaction with yields ranging from 13 to 95 %.
Further, investigations were made into possible generation of iminium ions via a transition metal catalyzed photoredox reaction and also into possible synthesis of BINOL-peptide phosphoric acids.
Finally, a number of enantiomerically enriched tetrahydrocarbazoles have been synthesized via a novel Friedel-Crafts-type cyclisation/ substitution reaction sequence. 4-(Indol-3-yl) butanal can undergo a 6-exo-trig cyclisation to form 2,3,4,9-tetrahydrocarbazol-1-ol. Under acidic conditions, the hydroxyl group can be substituted by an external indole nucleophile via a benzyl stabilized carbocation. The stereoselectivity of the reaction is dictated by a chiral phosphoric acid and enhanced by the presence of a bulky auxiliary attached to the nucleophile. The tetrahydrocarbazoles were isolated in 33-70 % yield and 18 to 97 % ee.