Efficient Short-cuts in Drug Discovery
Rather than a linear path of consecutive reactions, application of two or more simultaneous reactions can be more effective. Such tandem reactions can open new doors in drug discovery, and facilitate the total synthesis of complex molecules.
Synthesis of large and complex molecules in drug discovery and other organic chemistry involves multiple individual reactions. Traditionally these have been carried out in a linear fashion, where the product of one reaction becomes the starting material for the next. However, it would be faster and more efficient in terms of the resources needed if two or more reactions could be performed simultaneously. This has spurred an interest in tandem reactions and even total synthesis. The thesis contributes with new methodology in this field.
A total of five main contributions are presented.
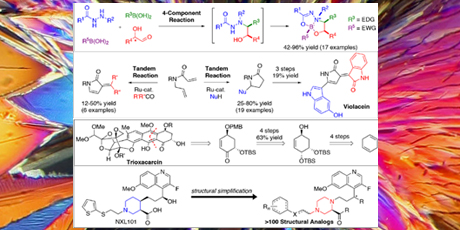
In the first project, a novel 4-component reaction of hydrazides, α-hydroxy aldehydes and two orthogonally reactive boronic acids, yielding distinct dioxadiazaborocines (DODA borocines) in a highly stereoselective fashion, has been developed. The products have yet to find an application, but may be interesting as pharmaceuticals or as inducers of stereoselectivity in enantioselective reactions.
Secondly, ruthenium-catalyzed tandem reactions combining ring-closing metathesis (RCM), isomerization of double bonds, and either aldol condensation or nucleophilic addition afforded a series of 5-substituted 2-pyrrolidinones and 3-substituted 2-pyrrolones.
Further, tandem methodologies provided a new entry to the total synthesis of the bioactive natural product violacein, which was synthesized in four steps with an overall yield of 13 %.
A fourth project investigated total synthesis of the antitumor antibiotic trioxacarcin. An efficient stereoselective route to a key cyclohexanone fragment was devised. The route featured an enantioselective epoxide rearrangement and proceeded in eight steps from 1,4-cyclohexadiene. This holds promising perspectives for research on trioxacarin structure-activity relationship and in drug discovery.
Finally, an efficient synthetic route to piperazine- based structural analogs of the antibiotic NXL101 has been developed. This substance has been withdrawn from clinical phase I trials due to an off-target interaction. In the project more than 100 structural analogs were synthesized and several of these showed promising antibiotic activity along with a reduced off-target interaction compared to NXL101.
Overall, the five projects have contributed with new knowledge on tandem and multicomponent methodologies and access to scaffolds of interest in drug discovery.