Chemical Biology of Dietary Fibers
Dietary fibers are known to benefit human health. Synthetic analogs of the natural fiber compounds are attractive for research purposes as their properties can be controlled.
Dietary fibers have gained considerable interest due to their proven beneficial effects on human health. Plant fibers are mainly located in the plant cell wall and consist of many complex and heterogeneous polysaccharides. A number of techniques have been developed for the study of these substances. However, these techniques demand well-defined and pure oligosaccharides. This is traditionally obtained by purification of natural fibers, but this process is highly timeconsuming. Therefore, chemical synthesis is currently regarded as superior. The thesis describes a new preparative synthesis method for building blocks related to one large type of dietary fibers, hemicellulose.
During the last decade a range of biotechnological methods have been developed for the study of dietary fibers, including specific monoclonal antibodies, which are a key tool in proteincarbohydrate interactions, enzyme-linked immunosorbent assay (ELISA), and carbohydrate micro-arrays. These all depend on acceptable quantities of specific and pure oligosaccharides. An alternative to isolation of oligosaccharides from the plant cell wall is chemical synthesis.
The thesis describes a new preparative synthesis method for d-xylose and d-xylobiose building blocks through carbohydrate interconversion of d-glucose and d-cellobiose. Both building blocks are intermediates for synthesis of polysaccharides found in arabinoxylans, which is one of the major dietary fiber types in the primary cell wall of a number of plants.
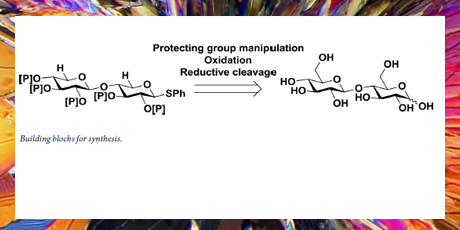
A partly protected d-cellobiose was obtained from cellobioside octaacetate in 37 % yield through eight optimized synthetic steps and subsequently oxidized to β–d-glucuronic acid-(1→4)-thio-β-d-glucuronic acid. The resul- ting diasaccharide was subjected to catalytic dehydrogenerative decarbonylation, where implementation of an in situ generated iridium catalyst gave the best results after optimization. Using a hydrogen scavenger in the presence of trace amounts of water, the desired xylobioside was achieved in good yield.
As proof-of-concept, the protected xylobioside glycosol donor was applied in the synthesis of oligoxylans, where an octasaccharide was assembled using only three glycosylation steps. Furthermore, the method proved to be an alternative route for selective equatorial deuterium labeling of d-xylose by retention of stereochemistry in the carbon-carbon bond scissoring step.