The polyesters cutin and suberin play key roles in protecting plants against external stress factors. Synthesizing these natural polymers in the lab is a gateway to new insight.
Plants produce a range of polymers, which are potential sources of renewable and biodegradable plastic. In the project, the approach of a synthesis chemist is applied in order to understand the biosynthesis of cutin and suberin, two of the most abundant natural polyesters, by synthesizing some of their monomers and studying their enzymatic polymerization in vitro.
All living organisms are covered by a layer of polymeric structural components, which defines their boundaries and serve as a barrier towards their environment. In the case of higher plants, especially two polyesters are instrumental in this protective function: cutin, located in the cuticle, which is found in the aerial parts of the plant, and suberin, in the underground parts and wound surfaces. Despite their highly important role in biological life, until recently little was known about the structure and biosynthesis of cutin and suberin.
Within the last decade, however, some light has been cast upon the subject. First, the essential role of glycerol-3-phosphate acyltransferases (GPAT) in cutin biosynthesis was discovered. GPATs selectively produce 2-mono-acylglyceryl esters (2-MAG) of fatty acid monomers. The most abundant monomer in tomato fruit (Solanum lycopersicum) cutin is 10,16-dihydroxyhexadecanoic acid. Its correspondent 2-MAG, 2-mono(10,16-dihydroxyhexadecanoyl)glycerol (2-MHG) was found in the soluble surface lipids of fruits carrying the cutin deficient 1 mutation.
Later a family of enzymes, the cutin synthases (CUS), were also found. CUS1 has shown in vitro activity towards the polymerization of 2-MHG, strengthening the hypothesis that cutin biosynthesis is extracellular and occurs through a series of transesterification reactions releasing glycerol.
In the project, cutin and suberin monomer derivatives were synthesized. Subsequently, in vitro oligomerization catalyzed by CUS1 was achieved.
Firstly, both deuterium and tritium-labelled 2-MHG were successfully synthesized. The deuteriumlabelled molecule was used in oligomerization assays together with 9-hydroxy 2-MHG to study the specificity of CUS1 towards the position of the mid-chain hydroxy group. CUS1 showed equal activity towards both monomers, suggesting that CUS1 is likely to participate in the incorporation of both monomers in cutin. Tritium-labelled 2-MHG could potentially be used to monitor transport and location of the monomer in planta.
Secondly, the synthesis and enzymatic oligomerization of 2-MAG derivatives of three fatty acids commonly found in suberin – behenic acid, ώ-hydroxy oleic acid, and octadecanedioic acid – was achieved. The three compounds were successfully synthesized and used as CUS1 substrates in enzymatic assays. The results suggest that enzymes from the CUS or another similar family could be involved in the biosynthesis of suberin.
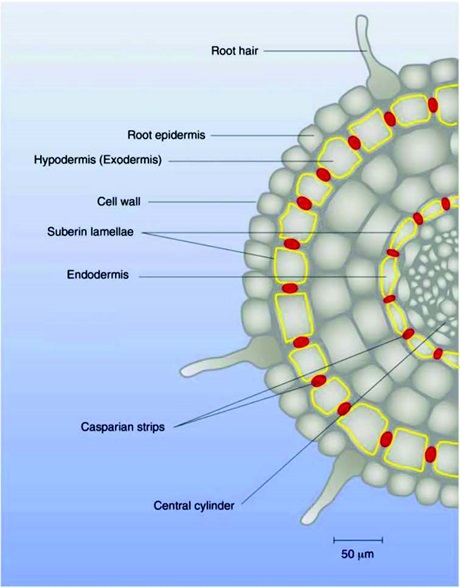
Transversal section of a root, showing the suberized exo- and endodermis cell walls as well as the casparian strips.