The project presents a new set of algorithms which are demonstrated to be effective for complicated systems in chemical industry and oil recovery.
Simultaneous calculation of chemical and phase equilibria (CPE) is highly relevant in the chemical industry, in oil and gas production, and in geochemistry. The project presents a new set of algorithms which are demonstrated to be robust and effective even for complicated systems.
CPE calculations are essential in demanding simulations of industrial processes. The applications include reactive distillation, heterogeneous organic synthesis, fuel synthesis from renewable feedstocks, and oil and gas production. Such calculations are also useful in association equation of state models, since association can be regarded as a type of reaction.
One class of CPE methods are the stoichiometric. While these are more intuitive, they are known to be less effective for systems involving many reactions. As complex challenges are the focus of this project, thus a non-stoichiometric approach was chosen.
The proposed solution is a hybrid of two different non-stoichiometric approaches and is therefore named “the combined method”. The first of the two methods, the Lagrange multipliers method, successive substitution is employed to solve a modified set of equations originating from the Lagrangian conditions at the minimum. The second method, the modified RAND method, one of the Lagrangian conditions is linearized around the current estimate of molenumbers. Composition derivatives of fugacity or activity coefficients are utilized to achieve quadratic convergence.
Combining the two methods improves robustness and efficiency. The Lagrange multipliers method isused for the first iterations of successive substitution, and the modified RAND method for final secondorder convergence. The combined algorithm has several advantages including a smaller system of equations (fewer variables), less sensitivity to initial estimates, the same treatment for all components and all phases, and the ability to monitor the decrease in Gibbs energy in the modified RAND step to guide convergence.
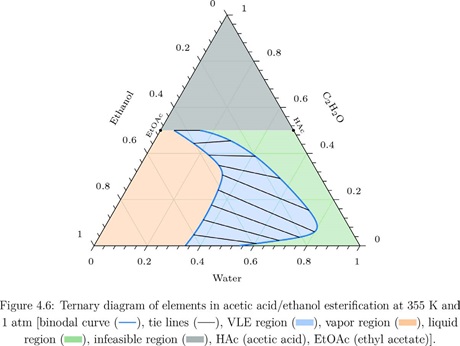
The combined method was applied to vapor-liquid, liquid-liquid, and vapor-liquid-liquid equilibrium of ideal as well as non-ideal systems for acid/alcohol esterifications, alkene/alcohol etherifications, hydration, hydrogenation, and isomer preparation. Additionally, predictions were made for the more complex transesterification of two individual triglycerides with methanol, which entails five chemical reactions and can result in one-, two-, or even three-phase equilibrium. Finally, CPE calculations were attempted for electrolyte systems, and the equilibrium solution was obtained for aqueous mixtures of electrolytes in contact with a vapor and a solid phase. As the method is robust to the presence of a solid phase, the algorithms are applicable even to more complicated geological systems with an electrolyte aqueous phase and multiple solids.
In summary, the method proved applicable all the way from simple one-reaction ideal systems to highly non-ideal electrolyte mixtures with speciation reactions and solids. Both algorithms were able to converge to the equilibrium solutions. Considering CPU time and the reasonable number of iterations, the method is demonstrated to be efficient and robust.
The project also involved a small study on dimethylether (DME) phase equilibrium modeling.