A manganese catalyzed cross-coupling reaction between aryl halides and Grignard agents is promising for green chemistry organic synthesis.
Still more chemists are faced with the task of producing fuel and other chemicals from non-fossil raw materials. Furthermore, they need to improve the sustainability of chemical processes by producing less waste. Catalysis is part of the solution to both problems. The project focusses on manganese catalyzed reactions for creation of carbon-carbon bonds.
Cross-coupling reactions which create a carbon-carbon bond are among the most important reactions in organic chemistry. The main focus of this project is the Kumada cross-coupling reaction which starts from a Grignard agent and an organic halide. When reported independently in 1972 by the groups of Robert Corriuand Makota Kumada, palladium (or nickel) catalyzed cross-coupling reaction was the first to be presented. The Kumada reaction continues to be used in a range of industrial applications such as the synthesis of drugs and manufacture of electronic components.
Palladium is a very versatile catalyst, but has the drawback of being both expensive and toxic. Therefore, finding good substitutes has become of interest. This project presents a MnCl2-catalyzed cross-coupling reaction between aryl halides and Grignard agents.
Aryl chlorides containing a cyano or an ester group in the para or ortho position react smoothly and in good yield. A variety of alkyl- and aryl-magnesium chlorides can be used in this cross-coupling reaction. The cross-coupling is believed to proceed by a SRN1 mechanism, and radical clock experiments were performed to elucidate this pathway. A tri-organomanganate complex is believed to be formed by the reaction between the organo-magnesium halide and manganese chloride, and it serves both as the nucleophile and the single electron donor. Other mechanistic hypotheses were excluded on the basis of the performed experiments.
An improved protocol was developed for the manganese catalyzed cross-coupling of two aryl-magnesium bromides under an atmosphere of dioxygen. The reaction is performed with a 2:1 ratio between the Grignard reagents and 20 % of MnCl2. When the limiting Grignard reagent undergoes little homo-coupling under the reaction conditions, very good yields of the hetero-coupling product can be achieved. Aryl-magnesium bromides with 4-methoxy, 4-dimethylamino, 4-fluoro, and 4-chloro substituents give high yields in the cross-coupling while heterocyclic Grignard reagents turned out to be poor substrates for the reaction.
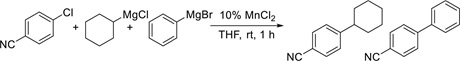
Reaction tested in the competitive time study.