Radio-iodinated liposomes have been developed with the aim of maximizing efficacy of local internal radio-therapy and minimizing side effects.
Localized internal radio-therapy offers the advantage of destroying primary solid tumors efficiently. However, it remains a challenge to find the optimal way to deliver the relevant isotopes in the body. The project presents remote loading strategies for incorporation of therapeutic compounds and contrast agents into gels and liposomes.
Radio-therapy is one of the most effective types of anti-cancer treatment. The aim is always to deliver the optimal dose to the tumor and the lowest possible dose to the organ at risk. To that end, local internal radio-therapy has emerged as an alternative to external beam radiation. For instance, brachy-therapy has proven successful in prostate cancer treatment, with very rare toxicity and a ten-year cancer-specific mortality of less than 5 %. This therapy relies on implantation of radioactive seeds nearby the cancer tissue, highlighting the importance of tumor-specific delivery and retention of radionuclides.
Over the last few decades liposomes have been investigated extensively as carriers for drug delivery. Liposomes are spherical, self-assembled structures formed by single or several concentric lipid bilayers with an aqueous phase inside and between the lipid bilayers. This structure enables them to entrap compounds. Several liposome systems are approved for drug delivery.
In the project, radio-iodinated liposomes were developed for radio-therapy and for molecular imaging to enable image guidance in therapy. Further, therapeutic radionuclide loaded injectable in situ forming depots were designed with the aim of minimizing side effects of radio-therapy and maximizing efficacy.
Firstly, the iodinated imaging agent diatrizoate was functionalized with a free amine to enable its remote loading into the stealth liposomes. An efficient remote loading method was developed for loading of liposomes with iodine and its radionuclides. Further, functionalized diatrizoate was radio-labeled with 125I and 124I to optimize the radiolabeling protocol and perform PET imaging. Following radio-labeling of the compound, the 124I-radiolabeled diatrizoate analogue ([124I]ADA) was remote-loaded into the stealth liposomes under optimized loading conditions. These liposomes were evaluated in mice in vivo quantifying the bio-distribution by PET/CT scanning. [124I]ADA showed prolonged blood circulation of 19.5 h and low accumulation in the spleen, liver, kidney, and tumor.
Also, injectable in situ forming depot formulations for the local delivery of therapeutic β-emitters, 177Lu and 90Y, in the tumor were investigated. A novel approach for brachy-therapy was proposed. 177Lu and 90Y were complexed with hydrophobic chelators to enable their controlled release from the depots or prolonged retention in the depot and tumor, and subsequent cellular internalization. The in vivo release of 177Lu was quantified by SPECT/CT imaging, and in vivo therapeutic efficacy of an optimized 177Lu depot was evaluated and tumor growth retardation up to eight days was observed. 90Y depots demonstrated even better anti-tumor efficacy, inhibiting tumor growth for twenty days. Also, the treated mice showed prolonged survival.
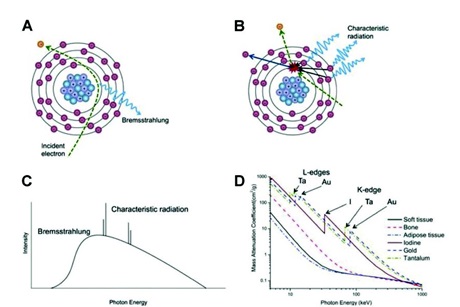
X-ray generation. A) Production of Bremsstrahlung. B) Production of characteristic radiation. C) X-ray emission spectrum showing Bremsstrahlung and characteristic radiation. D) Mass attenuation coefficient of some tissue and heavy elements as a function of X-ray energy.