The principle of SDS-Page is introduced in Wikepedia on this page.
This video from Bio-Rad describes how to run a SDS-Page experiment.
In our experiment we will be using the Bio-Rad system and precast SDS-Page gels (AnykD).
Protocol - Protein gel electrophoresis – SDS-Page
Sodium Dodecyl Sulfate – Poly Acrylamide Gel Electrophoresis (SDS-PAGE).
As acrylamide is toxic we are using pre-casted gels obtained from Bio-Rad. Use AnykD, 7.5% or 12% Tris-HCl gel and 2.6% crosslinker.
Preparation of samples
The preparation is carried out in a fume hood, due to the mercaptoethanol.
Turn on the heatblock, it should heat up to 80°C.
Just before use the sample buffer is prepared by adding 28µl beta-mercaptoethanol to an aliqout of 190µl 5x sample buffer.
The standard is prepared from 0.5µl standard (BIO RAD SDS-Page standard low range no. 161-0304) mixed with 19.5µl water.
Both standard and samples in aliqouts of 20µl is then added 5µl 5x sample buffer each, mixed and then heated to 80°C for 5-10 min and kept at room temperature prior to loading.
Centrifuge and mix thouroughly after heating, and mix again immedeatly before loading.
Preparation of gels
The gels are kept in the refrigerator at +4°C. When handling the gels wear gloves.
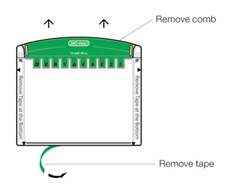 Remove the comb by pushing directly upward with your thumbs at the ends of the ridge and remove the tape at the bottom of the cassette (see picture).
Remove the comb by pushing directly upward with your thumbs at the ends of the ridge and remove the tape at the bottom of the cassette (see picture).
Then place the gel cassette in the holder with the wells facing inside. (If only one gel is used, the buffer dam is placed at the other side of the holder with the text facing inside as well).
The holder is placed in the electrophoresis cell, the poles marked with black and red on the holder is aligned with black and red color on the cell.
1x running buffer is poured outside the holder to the appropriate mark on the cell (either 2 or 4 gels) and filled inside the holder so it’s just above the top of the wells in the gel. By using a gel-loading pipette the wells in the gel are rinsed with running buffer before the samples are loaded.
Running conditions
Run the gel at 250V constant voltage until the blue color runs out of the bottom of the casette (approximately 20-25 minutes). To free the gel of the casette, gently twist the green metal pen between the two halfs of the casette at the black indicator arrows to break the casette.
Fix the gel for 30 min, stain for one hour and destain as desired, all three steps done at a strirring-table in the fume hood. Note that peptides are not completely fixed and may diffuse out of the gel if the fixing and staining times are greatly exceeded.
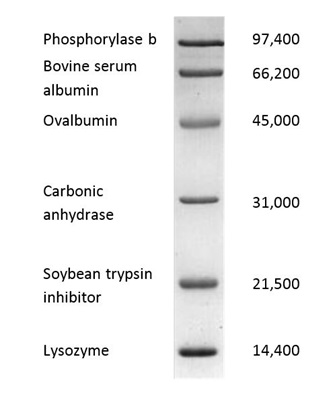
The SDS-Page standard low range no. 161-0304
Is mostly used as marker.

The picture shows how the broad range marker will distribute after a run.
Choose the gel with the overall best separation for your protein samples.
The gels available is 7,5%, 12% and AnykD.
Composition of buffers and solutions for running Tris-HCl gels.
Will be prepared by DTU Chemistry staff.
Make 1 L of 10x Running buffer and dilute to 1x before use
30.3 g Trismabase (final conc. in 1x running buffer is 25mM)
144 g Glycine (final conc. in 1x running buffer is 192mM)
10 g SDS (or 25ml 20% SD) (final conc. in 1x running buffer is 0.1%)
Dilute to 1000ml with water
Do not adjust pH
5x Sample buffer
200mM Tris/HCl pH 6.8, 2% SDS, 40% glycerol and 0.04% Coomassie G-250
2.0 ml water
2.0 ml 1.0M Trismabase/HCl pH 6.8
4.0 ml 100% glycerol
2.0 ml 10% SDS
4 mg Coomassie blue G-250
Just before use add 28µl beta-mercaptoethanol to an aliquot of 190µl 5x sample buffer.
Fixative solution
500 ml water
400 ml 96% ethanol
100 ml glacial acetic acid
Staining solution
900 ml water
100 ml glacial acetic acid
0.25 g Coomassie blue G-250 (use gloves and fume hood when handling solid)
The Coomassie blue dissolves quite slowly. Mix for one hour and filter through Whatman no. 1 filter paper or similar type.
Destaining solution
900 ml water
100 ml glacial acetic acid