Since the beginning of the 20th century, the interest for glucagon-like peptide-1 (GLP-1) has been growing. Firstly, due to GLP-1’s ability to regulate insulin secretion, and recently, due to its promising properties in treating obesity, preventing cardiovascular diseases, and possibly even dementia. However, it quickly became clear that GLP-1 cannot be administered as a drug, since its halflife in the body is very short. In fact, it is cleared already within a few minutes, preventing its usage as a drug without remedying modifications. Several strategies have been developed to successfully prolong the half-life of GLP-1. One solution consists of attaching a fatty acid (FA) chain to GLP-1. This is believed to cause interactions with human serum albumin (HSA), a carrier protein in the blood, as well as oligomerisation. However, it is vital that the biological function of GLP-1 is maintained.
In this study, the half-life extending properties of different attached FAs were investigated experimentally. The results show that longer FA chains lead to larger oligomers, and that the addition of a charged linker between GLP-1 and the FA chain leads to more stable, but smaller oligomers. In relation to HSA interactions, it was shown that attaching medium to long FA chains cause full interaction with HSA, whereas if there is a linker in between the long FA chains and GLP-1, only partial HSA interaction is observed. This concludes that longer FA chains should increase the half-life. But what about the biological effect? This was investigated using computer simulations, and the results indicate that the effect depends on the site of FA attachment. Furthermore, longer FA chains seemed to be more effective, which goes well with the half-life extension possibly being more pronounced for longer FA chains.
In addition, the pewrformed investigations, using a combination of experimental studies and computer simulations, could provide intel on a level that is very difficult to obtain using only one of the methods. Therefore, the results of this study also present themselves as a methodology for facilitating atomic level investigations of peptide drugs.
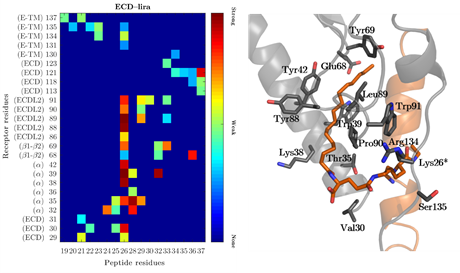
Interaction strength between peptide and receptor extracellular domain.