Protein and peptide therapeutics (PPTs), also called biologics, are often characterized by high specificity and potency with low toxicity and has therefore interested many pharmaceutical industries wanting to develop medicines to treat severe human diseases.
As a part of the consortium Protein-excipient Interactions and Protein-Protein Interactions in formulation (PIPPI) this PhD thesis describes fundamental research carried out to investigate the effect of pH and varying concentrations of different additives on diverse classes of PPTs. In the class of peptides, the wild-type plectasin – known as cysteine stabilized antimicrobial defensing – and three variants were investigated using molecular dynamics (MD) simulations in combination with microscale thermophoresis (MST) and nuclear magnetic resonance (NMR).
All the plectasin variants were conformationally stable during the course of 100 ns MD simulations at all pH and salt conditions. However, flexibility in the loop containing a distinct anionic tetrapeptide stretch close to the N-terminus increases with pH due to the change in electrostatics. The conformational stability of the plectasin variants are attributed to the presence of three cystines. Therefore, thermodynamic integration MD simulations supplemented with NMR chemical shift assays were used to determine the order of cystines reduction.
Peptide-excipient interaction hotspots were deduced from MD simulations in combination with MST and NMR measurements. In the classes of proteins, conformational stability of human serum transferrin (Tf) and conjugate fusion protein human serum albumin–neprilysin (HSA-NEP) were examined by combining small angle X-ray scattering (SAXS) and MD simulations in various formulation conditions.
The research findings presented in this thesis indicate the usefulness of combining in-silico and experimental techniques and that this approach can aid in designing new strategies for the formulation of biologics.
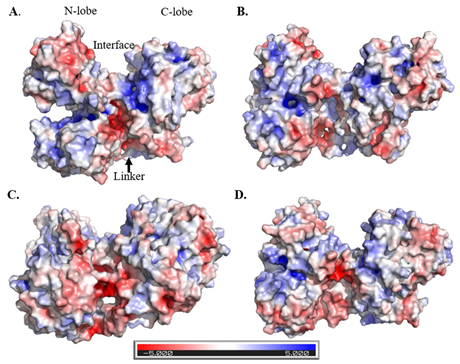
Electrostatic potential surfaces calculated for A. PO, pH 5; B. HO, pH 5; C. PO, pH 6.5; D. HO, pH 6.5.