Smarter Synthesis of Key Biological Components
The project presents smarter ways to create glycosylation, which is highly important in drug discovery and organic chemistry in general.
Oligosaccharides are a type of carbohydrates crucial to numerous biological processes. Included in the functions pertaining to oligosaccharides are the conformation, half-life and stability of proteins, antibodies, toxins, and cell signaling. Oligosaccharides consist of monosaccharides connected via glycosidic linkages. The reaction creating these linkages – glycosylation - is highly important in drug discovery and organic chemistry in general. The project presents smarter ways to create glycosylation.
Alterations in glycosylation patterns in many different proteins have been shown to result in change or loss of function for the protein. This has spurred great interest in improving the understanding of this biologically important process. A key tool in these investigations is synthesis of well-defined and pure carbohydrates and glyco-conjugates.
An important element of glycosylation synthesis is activation of glycosyl halides. The Koenigs-Knorr reaction has been the paramount coupling reaction for more than a century. However, in this and related procedures glycosyl halides are generally activated by metal salts, which most commonly contain silver or mercury. As these salts often are both toxic and expensive, it is attractive to create smarter pathways.
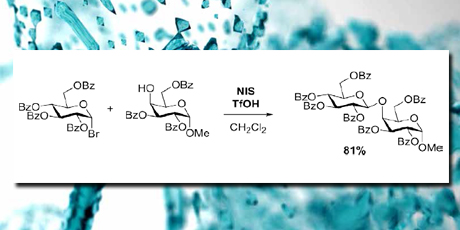
In the project, iodonium ions have been applied for activation of disarmed glycosyl bromides in the glycosylation of glycosyl acceptors. A method employing iodonium ions generated from N-iodosuccinimide and a protic acid was developed. The best results were obtained with the benzoyl protected glycosyl donors and acceptors. This method allows for the use of highly disarmed glycosyl bromides in a metal free glycosylation.
Further, a straightforward procedure for regioselective glycosylation of unprotected glycosyl acceptors using metal complexes was investigated. Here, disarmed glycosyl bromides and unprotected glycopyranoside acceptors were used.
Phenyl 1-thio-D-glycopyranosides and benzyl D-glycopyranosides derived from D-glucose, D-galactose, and D-mannose were treated with various metal complexes. Treatment with copper complexes followed by glycosylation did not yield any desired disaccharide products, however for tin, molybdenum and zirconium selective glycosylation of either the 3 or the 6 position was achieved. Acceptors derived from D-galactose underwent regioselective glycosylation of the 3 position in 51 % yield employing dibutyltin dichloride.
Molybdenum dioxide complexes facilitated a regioselective glycosylation of the 6 position for acceptors derived from D-glucose and D-mannose in 52 % and 35 % yield, respectively. Zirconium complexes achieved the best results with benzyl D-mannopyranoside acceptors resulting in 40 % yield of the 1,6-linked product.