Plant Cell Walls as Bioenergy Feedstock
Synthetic, well-defined substances are able to function as tools for investigations of key functions of the plant cells walls.
Biomass has great potential for sustainable production of chemicals and fuels and already contributes about 9-13 % to global energy supply. Unlike animal cells, the plant cells are surrounded by a wall, which provides a tough protective and supportive casting of the cell. The plant cell walls constitute almost half the biomass in plants. Thus, plant cell walls are a major target for biotech research. The project has developed synthetic, well-defined substances which are able to function as tools for investigations of key functions of the plant cells walls.
The plant cell wall is a highly organized composite of many different polysaccharides, proteins, and aromatic compounds. The diversity of polysaccharides makes structural studies challenging, as it is difficult to isolate well-defined fragments of specific polysaccharides simply from degradation of plant material. Chemical synthesis, on the other hand, is able to provide structurally diverse and well-defined oligosaccharides with excellent purity in large quantities.
Cellulose, the component present in plant cell wall in the largest quantity, was chosen as the focus of the project. To study the process of degradation of this polysaccharide, two non-natural substrates of cellohexaose were chosen as synthetic targets.
During natural degradation of plant cell walls, various enzymes play key roles. Structurally well-defined oligosaccharides made by chemical synthesis can be used as models for the more complex polysaccharides in investigation of properties such as polysaccharides biosynthesis, degradation and protein-carbohydrate interactions.
In the project two approaches for assembly of target molecules were applied. Initially, a 4-thio-acceptor was coupled with a PTFAIdonor. This afforded the product in a modest yield, in maximum 15 %.
A nucleophilic displacement of a leaving group with a 1-thiol improved the yield of the thio-linkage. The use of a thio-acetate, which was deacylated in situ, as a precursor to the thiolate, prevented the formation of the disulfide. This strategy relied on a convergent synthesis of both targets through two common building blocks: a S-trisaccharide and an O-trisaccharide. Both trisaccharides can be converted into a donor or an acceptor depending on the desired target. In this approach, a new acceptor was formed by a regioselective opening of a benzylidene acetal.
Finally, the activity of the glycosyltransferase GalAT was studied and partially characterized. The addition of a galacturonic acid residue onto a chemically synthesized fragment of RG-1 was investigated using crude microsomes containing Golgi membrane fragments from mung beans. An easy one-step assay was employed relying on the determination of the activity measured by luminescence. The optimal conditions were determined by varying pH, temperature and salt concentration.
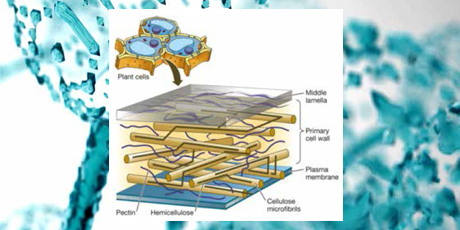
Illustration:
Schematic model of a segment of the primary plant cell wall.