Enzyme Substrates for Biomass Refining
Synthesis of model substrates for development of enzymes for processing of lignocellulose into bioethanol, paper, fine chemicals or other higher value products is presented.
The most abundant biomass source is lignocellulose, which is the non-edible part of plants. It can be an environmental problem when accumulated as waste from forestry, agriculture and agroindustry. In other words, lignocellulose is a cheap, renewable resource with the further advantage that it is not in demand by the food industry unlike most other biomass resources. The project presents synthesis of model substrates for development of enzymes that can enable the processing of lignocellulose into bioethanol, paper, fine chemicals or other higher value products.
Lignocellulose largely consists of a mixture of different, complex polymers. While the potential for lignocellulose as a sustainable resource is obvious, the practical applications are hampered by the intrinsic recalcitrance of these polymers to degradation. This is especially true for the so called lignin-carbohydrate complexes (LCCs), involving both hemicellulose residues and lignin.
Several physical, chemical, physicochemical and biological processes have been developed in the past decades to overcome this problem. Generally, the aim is to improve the enzyme digestibility. A key technology is the design of enzyme mixtures. An essential tool for biotechnology companies designing enzymes for biomass delignification is the access to well-defined model substrates. Understanding of the enzymes substrate specificity can be used to optimize enzyme mixtures towards natural, complex substrates. The chemically synthesized substrates often perform better in an industrial context than those isolated from natural sources.
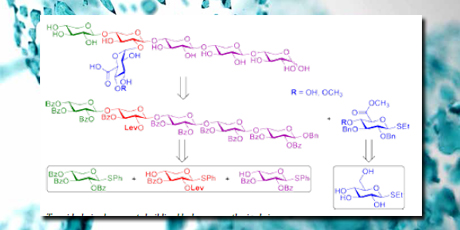
In the project, synthesis of two glucuronoxylan fragments was designed. Both fragments may serve as model substrates for the enzyme group xylanases. The synthesis involved the use of thioxyloside building blocks in an iterative, linear glycosylation strategy. A protectinggroup manipulation strategy was delveloped for the regioselective protection of the 2-position of the xylose residue on the fourth residue of (1→4)-β-pentasaccharide with a Lev group. The glycosylating procedure involved the use of the shelf-stable promoter p-NO2PhSCl and AgOTf. The length of the glycosyl donor, varying from a monosaccharide to a tetrasaccharide, did not affect the outcome. The method was effective and consistent for the type of substrates chosen and led to the desired pentasaccharide with a good 27 % overall yield.
In a second part of the project, three alkylaromatic and aromatic esters were prepared as mimics of lignin-carbohydrate complexes found in lignocellulosic biomass. These may serve as model substrates for the enzyme group glucuronoyl esterases. These esters have been used to characterize a novel glucuronoyl esterase from Cerrena unicolor (CuGE), produced by Novozymes, to obtain insight into the substrate specificity of the enzymes. HPLC analysis of the enzymatic reactions led to the determination of kinetic parameters that gave information about bonding affinity and catalytic efficiency.
In conclusion, the results suggest that glucuronoyl esterases could be effective on natural LCCs. This encourages further experiments aiming at future utilization in biomass delignification for forestry, feed and biofuel industries.
Illustration:
Two sidechain glucuronate building blocks were synthesized via a divergent strategy from the same thioethylglucose derivative.