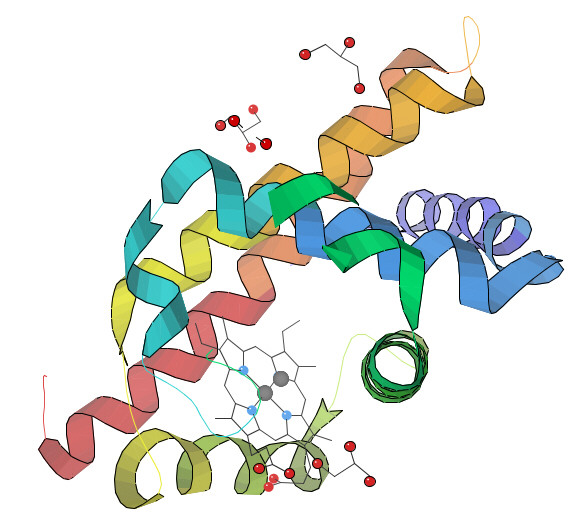
About 50% of all proteins are metalloproteins and their function are most often related to redox processes. The illustration above shows the structure of myoglobin. Due to limits in data resolution and the strong scattering from metal centres, the metal coordination will be poorly determined by X-ray crystallography. However, an accurate description of the redox centre in different oxidation and/or coordination states is the key to understand the mechanisms of such a protein.
In the last ten years EXAFS has significantly improved the situation. EXAFS is unique as it probes a specific element and reveals the oxidation state and will give detailed information of the metal centre coordination, typically with standard errors in distances better than 0.01 Å. EXAFS measurements can be performed on polycrystalline samples as well as on solutions. In case of polycrystalline samples EXAFS measurements can be combined with simultaneous powder diffraction to verify the polymorph and monitor changes of the overall global structure of the protein.
Bond lengths for the Fe-coordination in horse cytochrome can be determined very accurately by EXAFS.