X-ray Powder Diffraction (XRPD) applied on proteins is a relatively new method for characterization. Using high-resolution synchrotron radiation it is today possible to solve and refine smaller protein structures. Also conventional in-house X-ray powder diffraction has proven useful for polymorph identification and screening.
In many cases the growth of large single crystals is combined with many problems and seems to be a severe bottleneck in the structure determination of proteins. While the XRPD technique is well-developed for small molecules there seems to be more development challenges if this technique should be applied on proteins due to the inherent properties of macromolecules, e.g. high amounts of bulk-solvent.
The protein crystals are filled with disordered bulk-solvent located in the solvent channels of the structure. This is accounted for in the bulk-solvent correction.
Derivation of different correction models is used to calculate realistic XRPD patterns. The technique thus has high potential for identification. XRPD is also excellent to detect crystallinity, which may be valuable in crystallization optimization.
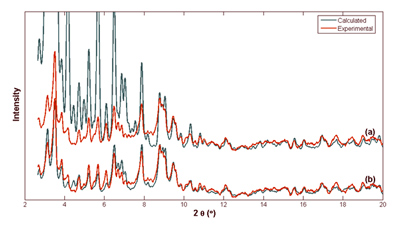
It is possible to calculate a realistic powder pattern, when correction models are applied.
(a) A non-corrected pattern of cubic insulin compared to an experimental.
(b) The same comparison after correction for bulk-solvent.