Successful Synthesis of a Plant Carbohydrate
The thesis presents strategies for chemical synthesis of linear and branched oligosaccharide fragments of rhamnogalacturonan I (RG I).
Pectin is the common name for a family of plant polysaccharides. Pectin plays an important role as a functional food ingredient, serving as a stabilizing and thickening agent in jam, jellies, yoghurts, fruit juice, and confectionary products. It is also used in biodegradable films, surface modifiers for medical devices, materials for biomedical implants, and for drug delivery. The wide range of practical applications has spurred interest in developing chemical synthesis of pectic oligosaccharides. This thesis contributes to this task.
Understanding pectin structure, function, and biosynthesis is essential for understanding, and potentially modifying cell wall structure. This can lead to new “designer” pectins with improved properties. Structurally defined oligosaccharide fragments of pectin can find a wide application for studying plant cell wall structure and function as well as plant cell wall acting enzymes. Pectic oligosaccharides can be obtained either by controlled chemical or enzymatic degradation of pectin followed by fractionation or by chemical synthesis. Of these methods, chemical synthesis promises production of structurally diverse oligosaccharides of excellent purity and in sufficient amount.
However, although modern carbohydrate chemistry has an extensive arsenal of methods to assemble virtually any oligosaccharide molecule, each case is an individual and often laborious task. Unlike in peptide and nucleic acid chemistry, in carbohydrate synthesis there is yet no universal approach.
The project focusses on rhamnogalacturonan I (RG I), which is one of the main structural classes of pectic polysaccharides. RG I is the second most abundant class of pectic polysaccharides. It has a complex chemical structure with a backbone of alternating α-(1→4)-linked L-rhamnose and α-(1→2)- linked D-galacturonic acid units with numerous branches of arabinans, galactans, or arabinogalactants positioned at C-4 of the rhamnose residues, with substantial structural variation within these branches.
The thesis presents strategies for chemical synthesis of linear and branched oligosaccharide fragments of RG I.
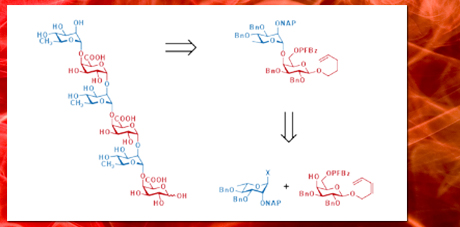
The first successful synthesis of a fully unprotected linear hexasaccharide fragment of the RG I backbone was accomplished. The strategy employs a highly modular approach which takes advantage of the armed-disarmed effect to generate the key n-pentenyl disaccharide donor in a chemoselective fashion.
Two protected n-pentenyl tetrasaccharide intermediates bearing the digalactan and the diarabinan side-chains have been synthesized. The suitably protected mono- and disaccharide donors have been utilized in the chemoselective glycosylations. The protective group pattern is designed to allow the assembly of larger branched RG I fragments.