A new initiative will help researchers at DTU Chemistry to generate market-ready innovations. After just one year, the initiative is creating results; a novel enzyme-cleavable linker with great medical perspectives is on steady course towards strong patents and multiple drug applications.
Denne artikel er fra DTU Kemi Årsrapport 2018. Læs den fulde rapport her.
Converting research into actual innovations can be somewhat of a challenge. Concerns about patents, which partners to team up with, and how to optimize test phases can be difficult to keep an overview of.
That is why DTU Chemistry, in 2018, initiated an innovation concept called Springboard.
At Springboard meetings, Department researchers can pitch innovative ideas and get direct feedback from industry partners.
“Springboard is a setup that guides researchers in the most efficient direction in terms of innovation. We link directly to the industry and have board members with many years of innovation experience who can advise researchers on every step of the way – from idea to product,” says Innovation Leader Thomas N. Kledal.
The Springboard currently consists of external board members Mads Laustsen, CMO at Symphogen, and Ole Bitsch-Jensen, Senior Partnership Manager at Coloplast. DTU Chemistry’s management and Innovation Officers Anders Riisager, Jane Pedersen and Thomas N. Kledal are also part of the board.
The external board members have signed confidentiality agreements, so Springboard participants are able to discuss research projects in details – without holding back information.
Thomas N. Kledal stresses that Springboard is not an isolated initiative, but a part of the Department’s overall innovation portfolio.
Linker in demand
At the first Springboard, one of the presenters was Assistant Professor Katrine Qvortrup.
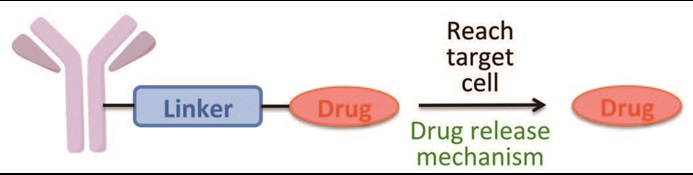
The Qvortrup Group at DTU Chemistry has developed a novel enzyme-cleavable linker technology for efficient and target-specific drug delivery. The flexibility in the design of the cleavable linker allows application in many different disease areas, including metabolic diseases, viral infections, and cancer.
According to Katrine Qvortrup, the chemical linker is able to release a wide variety of drugs, including alcohol-, amine-, and thiol-functionalized drugs. It is also very stable in plasma, which minimizes the drug side effects.
Even though the linker itself has great pharmaceutical potential, Katrine Qvortrupis convinced that Springboard acts as an important hub of knowledge when trying to commercialize an invention.
”Springboard evaluates your research through industrial glasses, so they know what to emphasize, throughout the entire innovation process. They give advice on which specific products your research is going to be held up against, and this is important to know in order to convince investors,” says Katrine Qvortrup.
Due to Springboard advices and the versatility of the linker, Katrine Qvortrup is already collaborating with multiple companies and research units.
Antag Therapeutics, for instance, are interested in the linker’s ability to target metabolic diseases by drug release in plasma at a controlled, predetermined rate. ADCendo and Rigshospitalet want to test the linkers in antibody drug conjugates, e.g. for breast cancer treatment. The company Synklino produces drugs treating viral infections, which make the company interested in phosphatase-cleavable linkers, since phosphatases are upregulated in viral infections. The highly modular design of the linker ensures that it can easily be modified to match the specific application, including enzyme and cleavage rate.
“In each of these collaborations, I need to have an overview of how to make the drug design process as efficient as possible, and Springboard offers a road map for this,” says Katrine Qvortrup.
Creating a stronghold
When your research has great application potential, patience can be the hardest virtue.
”As a young researcher, you want to see the research published,” Katrine Qvortrup points out. However, when it comes to developing new medicinal drugs, there is along way from basic research to production, and if you move too fast on a patent, it will most likely be weak.
“One of my main discussions with the Springboard was; what experiments do I need to conduct in order to secure the strongest patents – for all of the linker’s applications. They emphasized the importance of securing patents on full conjugates, not only the linker, because a patent on the linker alone would be vulnerable regarding drug development,” says Katrine Qvortrup.
This has led to more extensive testing of the modified linkers, creating a vast amount of preliminary data on specific medicinal drug conjugates.
Within a few months, in vitro experiments will be conducted at Finsen Laboratory at Rigshospitalet. The next steps in testing areal ready being talked through with the Phase I Unit at Rigshospitalet.
“As soon as we have strong in vitro data, we will apply for patents. Our patents will consequently be much stronger, benefitting both industrial partners and The Qvortrup Group,” she says.
Data led to spin-off project
In addition to the two Springboard events in 2018, the board offers ongoing correspondence and follow up meetings.
“It is key element to follow up on what has been discussed at the Springboard event itself. Innovation work does not stop, when the researcher steps out the door. It has to become a more integrated, natural element in the research process,” Innovation Leader Thomas N. Kledal says.
A correspondence with Mads Laustsen, CMO at Symphogen and external Springboard member, led Katrine Qvortrup to see existing data in a new light.
“Mads Laustsen asked if my group and I could develop a novel method to attach selective compounds on antibodies, due to market demands from the industry. We already had a lot of preliminary data on a new method, but did not recognize the true value of it. Now we do,” she says.
According to Katrine Qvortrup, the Springboard feedback regarding the novel method, at the follow up meeting, helped qualify her grant applications even more – one of them leading to a DKK 4.4 million grant from the Carlsberg Foundation.
“Funds are an essential driver of elevating your research. Therefore, having sparring partners with inside knowledge of the market and what investors look for is quite valuable for scientists,” she says.
About Antibody-drug conjugates (ADCs)
ADCs are comprised of three parts; a monoclonal antibody, a cytotoxic agent (the ‘drug’), and a structural moiety that joins the two together, the ‘linker’.
- Current linker technologies suffer from plasma instability, inefficient drug release, and limited selectivity.
- Katrine Qvortrup linker technology: Highly modular design – ease of synthesis and expeditious optimization of properties, incl. solubility, stability, enzyme-lability, selectivity, and release-rate.