Assoc. Professor (Lektor), PhD., D.Sc.
Research
Our main aim is to understand molecular properties and chemical reactivity via an atomic-level description based on the laws of physics. In short, the electronic structure of molecular systems determines the potential (i.e., forces) between atoms/nuclei and the methods for determining these forces via quantum mechanics (ab initio quantum chemistry or DFT) are already highly developed and implemented in computer programs.
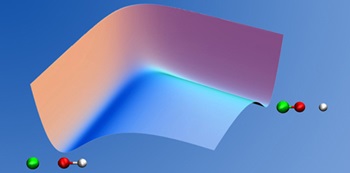
With known potentials, our focus is on the ultrafast atomic/nuclear motion described by quantum molecular dynamics. Such motion determines, e.g., the outcome of chemical reactions. Our work involves development of methods and concepts based on quantum mechanics and the implementation of relevant equations into computer programs.
The goals are, for example, to make contributions to our ability to predict the outcome of chemical reactions (including the calculation of reaction rate constants) and to provide new methods for controlling the outcome of such reactions.
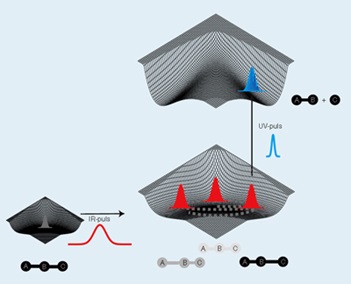
Written (together with F.Y. Hansen) the book: Theories of Molecular Reaction Dynamics: The microscopic foundation of chemical kinetics (Oxford University Press, 2008).
In July of 2013, we are organizing the Copenhagen Conference on Femtochemistry, Frontiers in Ultrafast Phenomena in Chemistry, Physics and Biology, see www.femto11.com
What happens when a molecule is hit by a flash of light or when two molecules collide ? These basic questions concern the detailed understanding of elementary chemical reactions. Chemistry - at the fundamental level - takes place within distances of a few atomic radii and within times as short as a few femtoseconds! Starting from a theoretical atomic-level description based on quantum dynamics, the two major areas of research are related to: (1) the prediction of the outcome of chemical reactions, including the calculation of the well-known thermal rate constant, and (2) the use of lasers in chemistry: (i) to create states of matter which cannot be created by normal thermal heating, (ii) to control - at the atomic scale - the transformation of matter, providing a fundamentally new way of controlling the outcome of chemical reactions going beyond traditional control using heat or catalysts, and (iii) to detect (”film”) atomic motion while chemical reactions take place.
The basic equation of motion for the theoretical description of nuclear/electronic motion in molecules –when they undergo collisions or are exposed to electromagnetic radiation – is the time-dependent Schrödinger equation for the non-stationary quantum dynamics on the relevant electronic potential energy surfaces.
Our research covers formal as well as computational studies with the aim of providing insights and methods of interest to all branches of chemistry. Key topics are:
Semi-classical description of nuclear motion. The numerical solution of the time-dependent Schrödinger equation is with the present computer technology limited to systems with a small number of degrees of freedom. Essential features of the nuclear motion can be described by (semi-) classical mechanics, e.g., based on the Wigner phase-space description of quantum mechanics or Gaussian wave packet dynamics. Semi-classical approaches provide also insights into quantum mechanical features like tunneling.
Molecule-surface collisions. Collisions between molecules and solid surfaces (catalysts) are an important step in heterogeneous catalysis. The theoretical study of such collisions allow, e.g. , for the determination of relevant rate constants.
Photofragmentation dynamics. Light-induced chemical reactions (from isomerization, fragmentation to photosynthesis) play an important role. We have, in particular, focused on theory and computation for photofragmentation dynamics. The study of these “half collisions” is related to many current experiments.
Detection and control of electron/nuclear dynamics (“atto- and femto-second chemistry”). The aim of atto-/femto-second chemistry is – in an interplay between theory and experiments – to detect and control the time evolution of molecular dynamics including chemical reactions – in real time on the atomic length scale. Our work related to the detection includes the theoretical foundation for pump-probe spectroscopy and for probing via ultrashort x-ray pulses. This work is closely related to the frontiers of experimental research, e.g., the new free-electron x-ray laser sources in Europe, Japan and USA.
In control beyond simple frequency tuning, laser excitation allows for coherent excitation of quantum states. This leads to creation of states of matter which cannot be created by traditional means. With the ongoing development of experimental techniques and laser technology, it is now possible to tailor laser pulses and hence to control quantum interference phenomena in molecules. We have, e.g., suggested schemes for the creation of non-stationary electronic states, for field-free orientation of molecules, and for selective bond breakage in polyatomic molecules.