As alkaloids are known to exert a wide variety of pharmacological effects, they have long been of great interest for numerous drug discovery projects. In a modern variation on a classical theme, a century-old chemical reaction for the synthesis of alkaloids has now been improved.
Morphine, cocaine, caffeine, nicotine, and ephedrine – all “household names” – are members of the alkaloid family, a group of naturally occurring compounds containing nitrogen atoms. Besides these well-known examples, a large variety of other alkaloids exist. Many of these occur in nature, while others are synthetic, typically produced as drug candidates inspired by the structures of biologically active natural products. Therefore, it is good news for drug developers that a group at DTU Chemistry has improved a key reaction for the synthesis of alkaloids.
In the classical reaction, discovered in 1911 by Amé Pictet and Theodor Spengler, an amine (for instance tryptamine) will undergo ring closure after condensation with an aldehyde to give important precursor molecules (for instance THBCs, tetra-hydro-β-carbolines) for the synthesis of alkaloids. Usually, a strongly acidic catalyst is employed and the reaction mixture is heated to promote the overall process. “The original Pictet-Spengler reaction can be highly efficient – or else it would not be still in use – but it has its drawbacks, since the heating and especially the strong acid represent rather harsh reaction conditions. Simple molecules can survive, but if you are looking to create more complex alkaloids, with more delicate chemical structures, you need a milder regime,” says Professor David Tanner, DTU Chemistry.
Offers mild conditions
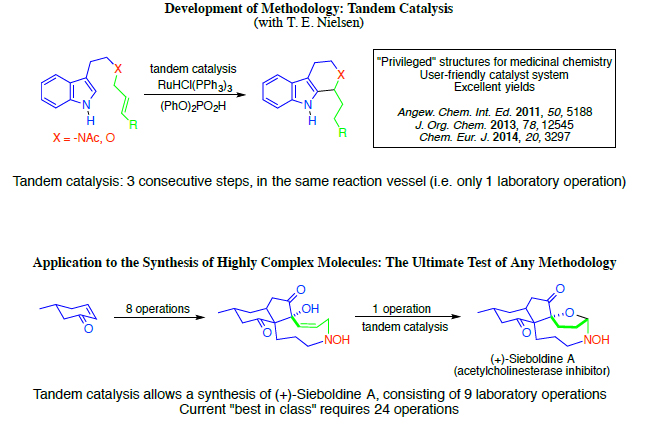
The new process produces 1,2,3,4-tetrahydro-β- carbolines (TBHCs) relying on the use of two chemically compatible catalysts, which are a ruthenium hydride complex and a Brønsted acid, respectively. The process is so-called tandem catalysis, meaning that both catalysts can simply be added to the reaction mixture in the same reaction vessel from the outset. Neither catalyst will interfere with the job of the other, as the effect of the Brønsted acid catalysis will set in only after the ruthenium hydride catalytic process has created the relevant compound for the other catalyst to work on.
“In other words, the synthesis can be performed as a one-pot process,” David Tanner under- lines. “We are not the first to create a milder alternative to the original Pictet-Spengler reaction, but we do think that the simplicity and user-friendliness of our solution is unique. For instance, our catalyst system is highly stable to air exposure and does not require work under glove-box conditions.” A further advantage of the new process is that, in contrast to the traditional reaction, it does not require the use of aldehydes as starting materials; the aldehydes required for the classical Pictet-Spengler reaction can sometimes be difficult to synthesise, and can also be quite unstable compounds. The modern variant uses alternative and more stable starting materials which are easy to produce.
“Also, the new reaction offers attractive possibilities for the total synthesis of structurally very intricate organic compounds, relying on the rapid generation of molecular complexity,” says David Tanner. “The art and science of total synthesis involves the development of synthetic methods which can efficiently transform simple starting materials into complex products.” From the simple to the complex In technical terms the process can be summarized as a ruthenium hydride/Brønsted acid catalyzed tandem isomerization / N-Acyliminium cyclization sequence for the synthesis of tetrahydro-β-carbolines. This means that three discrete chemical reactions can be carried out in a single laboratory operation, without the need to isolate and purify intermediates, thus increasing molecular complexity in a straightforward fashion.
The year 2011 was not only the International Year of Chemistry, but also the 100-year anniversary of the Pictet-Spengler reaction. The group at DTU Chemistry celebrated both events with a modern, milder version of the classical Pictet-Spengler reaction based on the experience in the Organic Chemistry area within novel catalytic techniques relying primarily on transition metal complexes. The idea was the brainchild of Professor Thomas E. Nielsen (now at Novo Nordisk), who suggested a ruthenium-catalyzed tandem sequence which efficiently transformed simple tryptamine derivatives into indolizinoindoles via N-acyliminium intermediates. “The project fitted well with the overall objective of our research, which can be summarized as finding ever more efficient ways to get from simple molecules to complex ones, which is an ongoing quest for synthetic organic chemistry,” David Tanner recalls.
“I was privileged to take part in the further development of the concept, in collaboration with Thomas E. Nielsen and some other exceptionally gifted younger colleagues.”
A superior solution
Two recent scientific publications present the current status of the work. The first article was published in the Journal of Organic Chemistry (JOC), 2013, presenting the tandem catalytic process. The second article, published in Chemistry, A European Journal, 2014, describes the synthesis of oxacyclic scaffolds (analogues of Pictet-Spengler products which contain oxygen atoms instead of nitrogen) via isomerization / cyclization of allylic ethers by employment of the same two catalysts. Together, the two articles confirm that the methodology can now be considered practically viable.
“First and foremost the process is relevant to fundamental research, and is of most interest to our colleagues in academia and in the drug discovery departments of the global pharmaceutical industry. These are the people who are faced with synthesis of the complex alkaloid precursors which our process is especially well suited for. However, in the long run it could well be imagined that the process will also be relevant for production of pharmaceuticals,” says David Tanner.