Transition metal catalysis has revolutionized organic chemistry and will be instrumental for providing food and energy to the growing global population. However, the current state-of-the-art solutions are too costly for these large-scale applications.
Photo: Shutterstock
The following article is from the DTU Chemistry Annual Report 2018. Read the full report here.
Almost like a magic touch, transition metal catalysis has entered organic chemistry, allowing reactions which were previously either extremely slow or simply impossible. Not only has this development been recognized with the chemistry Nobel prizes of 2001, 2005, and 2010; the implications for industry and society as such are large. As the global population continues to grow while fossil resources become exhausted, finding efficient ways to convert renewable feed stock into food, chemicals and fuel will be imperative. However, the current solutions rely mainly on expensive transition metals such as palladium or platinum.
“Presently, almost all really important transformations are catalyzed by precious elements. These metals have been the cornerstone of the field and responsible for much of the fundamental understanding of metal catalysis. Unfortunately, they are also extremely expensive,” says Professor Robert Madsen, DTU Chemistry.
Consequently, his group has embarked on a quest which will make the cheaper elements in the contingency of transition metals available.
Russia and South Africa dominate
Metal-catalyzed transformations are used around the globe every day for preparing pharmaceuticals, agrochemicals, vitamins, functional materials and many other applications. Obviously, a wide range of industrial corporations are keen to see their costly catalysts replaced by cheap ones. However,the benefits are far from only of an economic nature. The precious elements necessary incurrent solutions are only available in low quantities, and more than 80 per cent of the annual production comes from just two countries, Russia and South Africa.
“Thus, a key area of chemistry is based on precious metals with a very limited and potentially uncertain supply,” notes Robert Madsen. “It is therefore fair to say, that research aiming to develop Earth-abundant metals for homogeneous catalysis and elucidation of their reaction pathways is a substantial contribution to preserving our present-day standard of living.”
Strive to understand the mechanism
Two of the metals in focus are manganese and zinc. As both these elements are abundant and produced for other purposes, they are cheap. The current market price for both manganese and zinc is around 2 USD pr. kg – as compared to 37,900 and 28,700 USD pr. kg for palladium and platinum respectively.
“It is important for us not only to develop new and cheaper catalysts. We are also keen to understand the mechanisms, meaning there actions at the molecular level. This kind of understanding will provide a framework for rational decisions on which efforts are likely to lead to further improvements of the catalysts. We are not fans of the trial-and-error approach,” says Professor Robert Madsen.
“Typically, the cheaper alternatives do not follow the same mechanisms as the expensive platinum group metals do. This provides challenges, but is also very interesting from a scientific point of view, as we need to develop an entirely new basis for our work to succeed.”
In 2005, the group of Robert Madsen began developing benign catalytic procedures on alcohols and aldehydes by using ruthenium, iridium, and rhodium catalysts. In 2017, the group moved into novel catalysts based on Earth-abundant metals for dehydrogenating alcohols with the release of dihydrogen, i.e. R-CH2OH → R-CHO + H2.
“The carbonyl compound thus formed can be converted into other functional groups in the same transformation. This is an extremely competitive field and a hot topic in modern catalysis,” notes Robert Madsen.
Discovered a unique reactivity
Especially manganese catalysed (de)hydrogenations have received huge attention over the past two years. However, all the initial catalysts were expensive manganese(I) complexes stabilized by carbonmonoxide ligands. In 2018, the group at DTU Chemistry discovered a cheap manganese(III) salen complex able to catalyze the same transformation. In addition, the mechanism was elucidated and shown to involve the ligand. This constitutes the first example of a manganese(III) catalyst for releasing dihydrogen. In unpublished work, the group has also shown that a manganese(III) porphyrin complex catalyses the same transformation although the mechanism is not known.
“These discoveries show that manganese(III) complexes with tetradentate ligands display a unique reactivity in reactions with dihydrogen – something previously completely unknown,” according to Robert Madsen.
Other recent achievements by Robert Madsen and his colleagues are zinc oxide catalyzed dehydrogenation of primary alcohols into carboxylic acids, and manganese-catalyzed cross-coupling of arylhalides and Grignard agents.
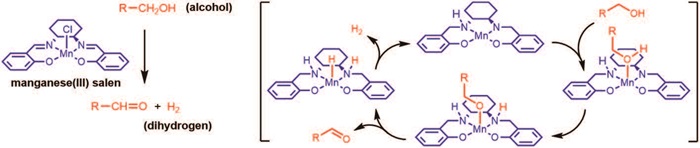
In 2018, the Robert Madsen Group at DTU Chemistry discovered a cheap manganese(III) salen complex able to catalyze (de)hydrogenations. This constitutes the first example of a manganese(III) catalyst for releasing dihydrogen.