A new compound of K
2MoO
2(SO
4)
2 was prepared by dissolving of MoO
3 in molten K
2S
2O
7 at 420 °C.
Raman studies of the melt was used to determine the overall reaction of the involved chemicals. The glassy melt was treated by cooling and subsequent heating to 280 °C where spherolitic crystilization occurred. The structure was determined by single-crystal analysis and further characterisation included FT-IR studies, powderdiffraction and Crystal Raman spectroscopy. The compound crystallizes in the monoclinic space group P21/c (No. 16) with a= 9,0144(3), b=12,4540(4), c=8,8874(3) and b=112,1940 (se upper figure). A Calibration study of the DILOR Raman apparatus was performed. Regular imperfections on the Spindle of the Sinusarm drive were determined by a newly developed procedure to be the cause of serious shifts in recorded spectras. A measuring procedure using certain "golden values" as scanning center for minimizing these shifts is presented.
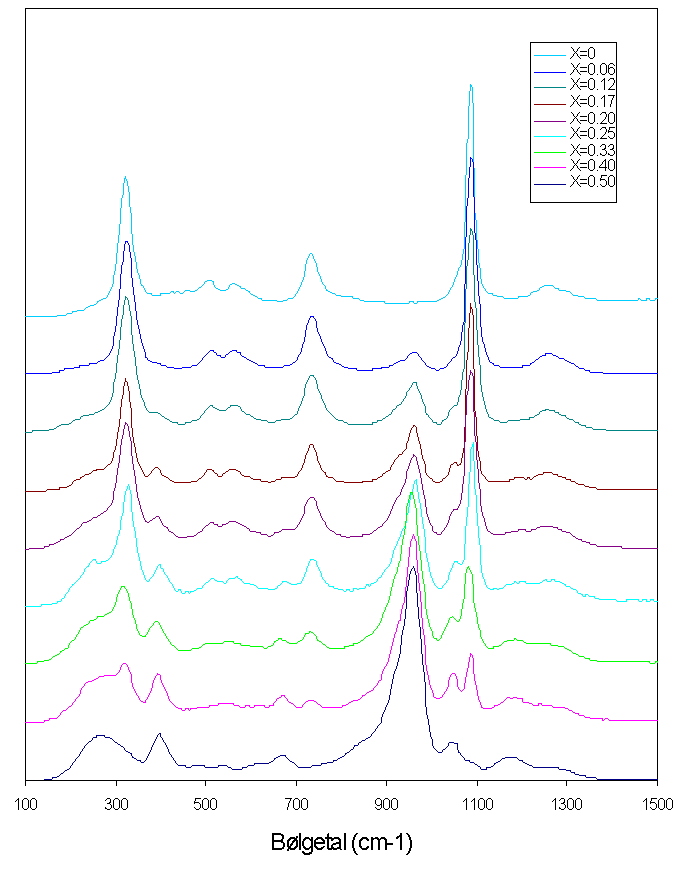
Figure: Smelte-Raman spektre for blandinger af MoO3 og K2S2O7 målt ved 430°C. Molbrøken af MoO3 er angivet i øverste højre hjørne.
References for K2MoO2(SO4)2
T. Nørbygaard, R.W. Berg and K. Nielsen. "The Reactions between MoO3 and Molten K2S2O7 forming K2MoO2(SO4)2, studied by Raman and IR Spectroscopy and X-ray Crystal Structure Determination".193rd Electrochem. Soc. Meeting, San Diego, USA, May 3-8, 1998. The Electrochem. Soc. Meetings Abstracts Vol. 98-1, Abstr. No. 1096, 1998. Proceedings - Electrochemical Society (1998), 98-11 (Symposium on Molten Salts XI), 553-573, Pennington, N.J.
S. Boghosian and R.W. Berg: "Determination of Stoichiometry of Solutes in Molten Salt Solvents by Correlations of Relative Raman Band Intensities". Appl. Spectrosc. 53 (5) (1999) 565-571.
A. G. Kalampounias, G. Tsilomelekis, R.W. Berg and S. Boghosian, Molybdenum (VI) Oxosulfato Complexes in MoO3–K2S2O7–K2SO4 Molten Mixtures: Stoichiometry, Vibrational Properties and Molecular Structures, J. Phys. Chem. A 116 (2012) 8861-8872.