Beam time is in high demand at the exclusive international facilities able to “film” on the femtosecond scale which applies to chemical reactions. A successful DTU modeling tool opens the doors.
Certain molecules are able to absorb light and use it internally to break or make chemical bonds. A team of scientists from DTU Chemistry and DTU Physics has successfully used advanced computer simulations to describe how one such molecule absorbs light, leading to a different structure of the complex and to a further dissipation of the energy. The findings were accepted for publication by the Journal of Physical Chemistry Letters, one of the most prestigious journals of the field. Further has the modeling tool, which made the accurate description possible, proven to be a ticket to an exclusive world of cutting edge science.
“We are not the first to observe this type of phenomena in a molecule. What is so special about the study is the amount of statistical information we have been able to generate. Our article contains about ten times more dynamic information compared to other articles. This enables us to not only “see something”, but also to perform large evidence-based simulations, which establish the prevalence of the various states of the molecule,” says Associate Professor Klaus B. Møller, DTU Chemistry.
The new tool developed by scientists from DTU Chemistry and DTU Physics builds on Quantum- /Molecular-Mechanical (QM/MM) Direct Dynamics. It describes excess excitation energy dissipation via dynamic changes in molecular structure, vibrations and solvation.
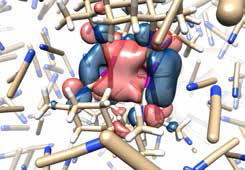
Snapshot of a simulation made with the new theoretical tool developed by the DTU-scientists. The molecule in the middle is treated with quantum mechanics, under the influence of the classically described solvent particles.
Filming a chemical reaction
Traditionally a chemical reaction is described by an equation which features the reactants on the left hand side and end products to the right. This picture is, however, simplified as even simple reactions involve a set of sub-reactions so complex that it would take vast amounts of modelling and computer calculations to describe them. But recently advanced international facilities has been built in which it is possible to “film” chemical reactions using X-rays.
A chemical reaction can be compared to a football game in the sense that only in a fraction of the total time consumed will the events that are most important to the spectator – the actual scoring of goals – take place. The vast majority of time is used for getting the conditions for scoring a goal in place. Once the conditions are right, it will only take seconds to score the football goal – and in parallel; the transformation of a molecule takes just a few hundred femtoseconds (one femtosecond is 10-15 second). Hence the field has been dubbed femtochemistry.
The new advanced facilities are able to generate X-ray pulses so short that processes at this incredibly small time scale may be seen.
The three main facilities of interest to the DTU team are the Linac Coherent Light Source (LCLS), operated by Stanford University, USA, the Japanese X-ray laser system SACLA, and the soon-to-open European facility, XFEL, in Hamburg, Germany.
'Any result will be first of its kind'
Postdoc Asmus Ougaard Dohn, DTU Chemistry, has been involved in experiments at both LCLS and SACLA during his recently concluded PhD project. The experiments included the Iridium- containing complex which is the basis for the Journal of Physical Chemistry Letters article.
“It is a real privilege to work at such cutting edge facilities,” Asmus Ougaard Dohn remarks. “It is a privilege and a challenge: We more or less need to build the analytical tools from scratch, to achieve results. But almost every result we get will be the first of its kind.”
The studied complex is [Ir2(dimen)4]2+. This binuclear Iridium-containing complex was chosen mainly for two reasons. Firstly, it is a large complex containing heavy atoms with a high number of electrons, which makes it easier to observe and get statistically viable results. Secondly, it has been studied by several other groups, including collaborators at Stanford University. This provides a background for validating the results.
Even though the complex had been studied by several other groups, the DTU-scientists were able to provide new insight. The molecule was believed to exist in two main distinct states, but the study revealed a previously unknown third state. The group has dubbed this the “breathing” state, as here the molecule alters its structure periodically, much like a person breathing. Another new insight feature involves the relation between the molecule and the solvent utilized. Traditionally the solvent is not seen as a part of the experiment but here the dissemination of energy from the complex to the solvent was followed closely. Surprisingly, the solvent was seen to stabilize the molecule leading to a longer period of concerted motion – in contrast to the general assumption.
Relevant to solar cell applications
While these discoveries have credited the team in the academic world, it is still a long route to practical applications.
“Molecular absorption of light and the precise resulting distribution of energy are in fact of interest to a number of applications. One of these would be solar cells. It is very likely that an improved understanding of such processes can lead to significantly better photovoltaic cells. However, the Iridium-containing complex which we studied will never be seen in such cells. It is a model molecule with mainly basic scientific importance,” says Asmus Ougaard Dohn.
“We are enthusiastic about the four beam time sessions we will have in 2015 and also by the fact that several international groups have invited us to take part in their projects. In other words, they invite us to do calculations based on their experiments because they know we can contribute,” notes Klaus B. Møller.