Untitled Document
Our main aim is to understand molecular properties and chemical reactivity via an atomic-level description based on the laws of physics. In short, the electronic structure of molecular systems determines the potential (i.e., forces) between atoms/nuclei and the methods for determining these forces via quantum mechanics (ab initio quantum chemistry or DFT) are already highly developed and implemented in computer programs.
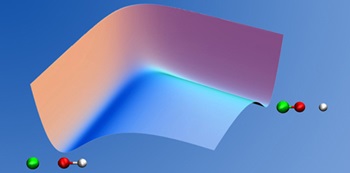
With known potentials, our focus is on the ultrafast atomic/nuclear motion described by quantum molecular dynamics. Such motion determines, e.g., the outcome of chemical reactions. The basic equation of motion for the theoretical description of nuclear/electronic motion in molecules –when they undergo collisions or are exposed to electromagnetic radiation – is the time-dependent Schrödinger equation for the non-stationary quantum dynamics on the relevant electronic potential energy surfaces. Our work involves development of methods and concepts based on quantum mechanics and the implementation of relevant equations into computer programs.
The goals are, for example, to make contributions to our ability to predict the outcome of chemical reactions (including the calculation of reaction rate constants) and to provide new methods for controlling the outcome of such reactions.
Written (together with F.Y. Hansen) the book: Theories of Molecular Reaction Dynamics: The microscopic foundation of chemical kinetics (Oxford University Press, 2008).
In July of 2013, we organized the Copenhagen Conference on Femtochemistry, Frontiers in Ultrafast Phenomena in Chemistry, Physics and Biology.
Our research covers formal as well as computational studies with the aim of providing insights and methods of interest to all branches of chemistry. Key topics are:
- The prediction of the outcome of chemical reactions, including the calculation of the well-known thermal rate constant
-
The use of lasers in chemistry:
- to create states of matter which cannot be created by normal thermal heating
-
to control - at the atomic scale - the transformation of matter, providing a fundamentally new way of controlling the outcome of chemical reactions going beyond traditional control using heat or catalysts
-
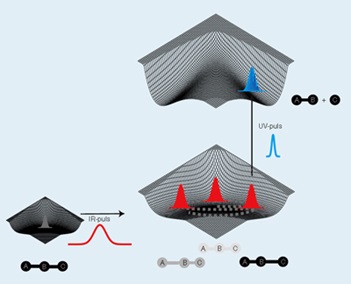
to detect (”film”) atomic motion while chemical reactions take place.
Further details: